Animal NDC 57926-720-61 P.g. 600
Gonadotrophin, Chorionic And Choriogonadotropin Alfa
Animal Product Information
P.g. 600 Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
Other
(serum gonadotropin and chorionic gonadotropin for injection)
Approved by FDA under NADA # 140-856
FOR ANIMAL USE ONLY
INDICATIONS FOR USE
PREPUBERAL GILTS: P.G. 600 is indicated for induction of fertile estrus (heat) in healthy prepuberal (non-cycling) gilts over five and one-half months of age and weighing at least 85 kg (187 lb.).
SOWS AT WEANING: P.G. 600 is indicated for induction of estrus in healthy weaned sows experiencing delayed return to estrus.
DIRECTIONS FOR USE
FIVE DOSE VIAL: Using a sterile syringe and a sterile 0.90 × 38 mm (20 G × 1½") hypodermic needle, transfer approximately 5 mL of the sterile diluent into the vial of freeze-dried powder. Shake gently to dissolve the powder. Transfer the dissolved product back into the vial of diluent and shake gently to mix. Inject one dose (5 mL) of the reconstituted solution into the gilt's or sow's neck subcutaneously behind the ear.
398967 R2 INU240 03
Manufactured for:
Intervet Inc., a subsidiary of Merck & Co. Inc.
Madison, NJ, 07940 USA
by:
INTERVET INTERNATIONAL GmbH
UNTERSCHLEISSHEIM, GERMANY
Rev. 10/2022
MERCK
Animal Health
Description
DESCRIPTION
Gilts normally reach puberty (begin experiencing normal estrous cycles and exhibiting regular estrus or heat) at any time between six and eight months of age, although some gilts will not have exhibited their first estrus at ten months of age. Age at first estrus is influenced by several factors including breed type, season of the year, environmental conditions, and management practice (Hurtgen, 1986).
Sows normally exhibit estrus three to seven days after weaning their litters; however, some otherwise healthy sows may not exhibit estrus for 30 days or more after weaning (Dial and Britt, 1986). The causes of delayed return to estrus in healthy sows are poorly understood, but probably include season of the year (so-called seasonal anestrus; Hurtgen, 1979), adverse environmental conditions, such as high ambient temperatures (Love, 1978), and the number of previous litters, because the condition is more prevalent after the first litter than after later litters (Hurtgen, 1986).
P.G. 600 (serum gonadotropin and chorionic gonadotropin for injection)is a combination of serum gonadotropin (Pregnant Mare Serum Gonadotropin or PMSG) and chorionic gonadotropin (Human Chorionic Gonadotropin or HCG) for use in prepuberal gilts (gilts that have not yet exhibited their first estrus) and in sows at weaning. It is supplied in freeze-dried form with sterile diluent for reconstitution.
In gilts and sows, the action of serum gonadotropin is similar to the action of Follicle-Stimulating Hormone (FSH), which is produced by the animals' anterior pituitary gland. It stimulates the follicles of the ovaries to produce mature ova (eggs), and it promotes the outward signs of estrus (heat).
The action of chorionic gonadotropin in gilts and sows is similar to the action of Luteinizing Hormone (LH), which is also produced by the animals' anterior pituitary gland. It causes the release of mature ova from the follicles of the ovaries (ovulation), and it promotes the formation of corpora lutea, which are necessary for the maintenance of pregnancy once the animals have become pregnant.
The combination of serum gonadotropin and chorionic gonadotropin in P.G. 600 induces fertile estrus in most prepuberal gilts and weaned sows three to seven days after administration (Schilling and Cerne, 1972; Britt et al., 1986; Bates et al., 1991). The animals may then be mated or, in the case of gilts, mating may be delayed until the second estrus after treatment.
NOTE: P.G. 600 IS INTENDED AS A MANAGEMENT TOOL TO IMPROVE REPRODUCTIVE EFFICIENCY IN SWINE PRODUCTION OPERATIONS. TO OBTAIN MAXIMUM BENEFIT FROM THIS PRODUCT, ESTRUS DETECTION AND OTHER ASPECTS OF REPRODUCTIVE MANAGEMENT MUST BE ADEQUATE. IF YOU ARE IN DOUBT ABOUT THE ADEQUACY OF YOUR BREEDING PROGRAM, CONSULT YOUR VETERINARIAN.
How Supplied
P.G. 600 is available in:
FIVE DOSE VIALS (order Code No. PG-720-5) - One vial containing white freezedried powder, and one vial containing sterile diluent. When reconstituted, the five dose vial (25 mL) of P.G. 600 contains:
| SERUM GONADOTROPIN (PMSG) | 2,000 IU |
| CHORIONIC GONADOTROPIN (HCG) | 1,000 IU |
| equivalent to 1,000 USP Units chorionic gonadotropin) | |
Warnings
CAUTIONS
Treatment will not induce estrus in gilts that have already reached puberty (begun to cycle). Gilts that are less than five and one-half months of age or that weigh less than 85 kg (187 lb.) may not be mature enough to continue normal estrus cycles or maintain a normal pregnancy to full term after treatment.
Treatment will not induce estrus in sows that are returning to estrus normally three to seven days after weaning. Delayed return to estrus is most prevalent after the first litter; the effectiveness of P.G. 600 has not been established after later litters. Delayed return to estrus often occurs during periods of adverse environmental conditions, and sows mated under such conditions may farrow smaller than normal litters.
Dosage & Administration
DOSAGE AND ADMINISTRATION
One dose (5 mL) of reconstituted P.G. 600, containing 400 IU serum gonadotropin (PMSG) and 200 IU chorionic gonadotropin (HCG), should be injected subcutaneously into the gilt's or sow's neck behind the ear.
Prepuberal gilts should be injected when they are selected for addition to the breeding herd. Sows should be injected at weaning during periods of delayed return to estrus.
Storage And Handling
STORAGE PRECAUTIONS
Store at 36-46°F (2-8°C).
Once reconstituted, P.G. 600 should be used immediately. Unused solution should be disposed of properly and not stored for future use.
Spent hypodermic needles and syringes generated as a result of the use of this product must be disposed of properly in accordance with all applicable Federal, State and local regulations.
References
REFERENCES
Bates, R.O., B.N. Day, J.H. Britt, L.K. Clark and M.A. Brauer (1991). Reproductive performance of sows treated with a combination of Pregnant Mare's Serum Gonadotropin and Human Chorionic Gonadotropin at weaning in the summer. Journal of Animal Science 69:894.
Britt, J.H., B.N. Day, S.K. Webel and M.A. Brauer (1989). Induction of fertile estrus in prepuberal gilts by treatment with a combination of Pregnant Mare's Serum Gonadotropin and Human Chorionic Gonadotropin. Journal of Animal Science 67:1148.
Dial, G.D., and J.H. Britt (1986). The clinical endocrinology of reproduction in the pig. In: D.A. Morrow (ed.). Current Therapy in Theriogenology 2. W.B. Sanders Company, Philadelphia. p. 905.
Hurtgen, J.P. (1979). Seasonal breeding patterns in female swine. Ph.D. Dissertation, University of Minnesota.
Hurtgen, J.P. (1986). Noninfectious infertility in swine. In: D.A. Morrow (ed.) Current Therapy in Theriogenology 2. W.B. Sanders Company, Philadelphia. p. 962.
Love, R.J. (1978). Definition of a seasonal infertility problem in pigs. Veterinary Record 103:443.
Schilling, E., and F. Cerne (1972). Induction and synchronization of oestrus in prepubertal gilts and anoestrus sows by a PMS/HCG-compound. Veterinary Record 91:471.
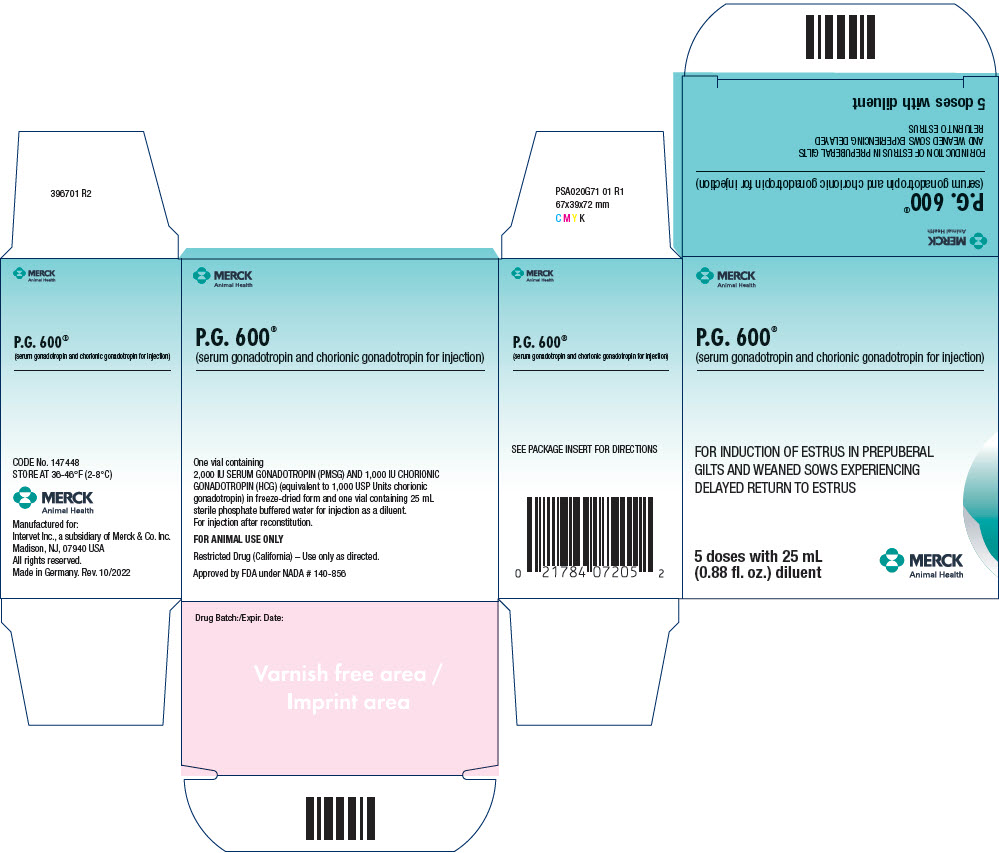
Principal Display Panel - 25 Ml Multi-Dose Vial Carton
MERCK
Animal Health
P.G. 600®
(serum gonadotropin and chorionic gonadotropin for injection)
FOR INDUCTION OF ESTRUS IN PREPUBERAL
GILTS AND WEANED SOWS EXPERIENCING
DELAYED RETURN TO ESTRUS
5 doses with 25 mL
(0.88 fl. oz.) diluent
MERCK
Animal Health
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.