Animal NDC 10797-959-01 Adequan I.m.
Polysulfated Glycosaminoglycan
Animal Product Information
Adequan I.m. Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
Description:
Each mL contains Polysulfated Glycosaminoglycan (PSGAG) 100 mg, Benzyl Alcohol 0.9% v/v as a preservative, and Water for Injection q.s. Sodium Hydroxide and/or Hydrochloric Acid added when necessary to adjust pH. The solution is clear, colorless to slightly yellow.
Pharmacology:
Polysulfated Glycosaminoglycan is chemically similar to the glycosaminoglycans in articular cartilage matrix. PSGAG is a potent proteolytic enzyme inhibitor and diminishes or reverses the pathologic processes of traumatic or degenerative joint disease which result in a net loss of cartilage matrix components. PSGAG improves joint function by reducing synovial fluid protein levels and increasing synovial fluid hyaluronic acid concentration in traumatized equine carpal and hock joints.
Indications:
Adequan® i.m. Multi-Dose is recommended for the intramuscular treatment of non-infectious degenerative and/or traumatic joint dysfunction and associated lameness of the carpal and hock joints in horses.
Dosage And Administration:
Practice aseptic techniques in withdrawing each dose to decrease the possibility of post-injection bacterial infections. Adequately clean and disinfect the stopper prior to entry with a sterile needle and syringe. Use only sterile needles, and use each needle only once.
The vial stopper may be punctured a maximum of 10 times.
The recommended dose of Adequan® i.m. Multi-Dose in horses is 500 mg every 4 days for 28 days intramuscularly. The injection site must be thoroughly cleansed prior to injection. Do not mix Adequan® i.m. Multi-Dose with other drugs or solvents.
Contraindications:
There are no known contraindications to the use of intramuscular Polysulfated Glycosaminoglycan.
Warnings:
Do not use in horses intended for human consumption. Not for use in humans. Keep this and all medications out of the reach of children.
Precautions:
The safe use of Adequan® i.m. Multi-Dose in horses used for breeding purposes, during pregnancy, or in lactating mares has not been evaluated.
Safety And Efficacy:
Safety and efficacy studies utilizing Adequan® i.m. Multi-Dose were not performed. Adequan® i.m. Multi-Dose was approved based on the conclusion that the safety and effectiveness of Adequan® i.m. Multi-Dose will not differ from that demonstrated for the original formulation of Adequan® i.m.
Animal Safety:
Animal Safety studies utilizing Adequan® i.m. Multi-Dose were not performed. Safety studies were conducted in horses using the single dose formulation. Doses as high as 2,500 mg were administered intramuscularly to 6 horses twice a week for 12 weeks. This dosage is 5 times the recommended dosage and 3 times the recommended therapeutic regimen. Clinical observations revealed no soreness or swelling at the injection site or in the affected joint. No animal had any clinical or laboratory evidence of toxicity.
Storage Conditions:
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) (See USP Controlled Room Temperature). Avoid prolonged exposure to temperatures ≥ 40°C (104°F).
Use within 28 days of first puncture and puncture a maximum of 10 times. Dispose of spent needles in accordance with all federal, state and local environmental laws.
How Supplied:
Adequan® i.m. Multi-Dose solution, 5,0000 mg/50 mL (100 mg/mL) in 50 mL multi-dose glass vials.
NDC 10797-959-01 50 mL Multi-Dose Vials Packaged 1 vial per box
AMERICAN REGENT, INC.
ANIMAL HEALTH
Shirley, NY 11967
(1-888-354-4857)
Made in U.S.A.
IN959
Rev. 9/2021
MG# 44453
Approved by FDA under NADA #140-901
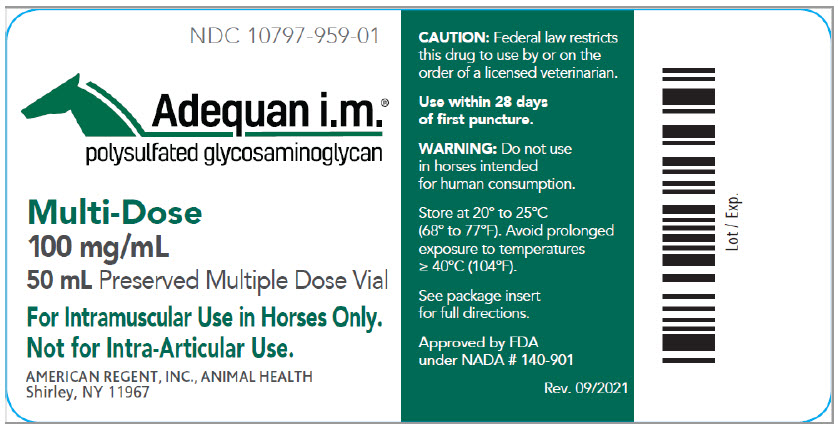
Container Label
NDC 10797-959-01
Adequan i.m.®
polysulfated glycosaminoglycan
Multi-Dose
100 mg/mL
50 mL Preserved Multiple Dose Vial
For Intramuscular Use in Horses Only. Not for Intra-Articular Use.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Use within 28 days of first puncture.
WARNING: Do not use in horses intended for human consumption.
Store at 20° to 25°C (68° to 77°F). Avoid prolonged exposure to temperatures ≥ 40°C (104°F).
See package insert for full directions.
AMERICAN REGENT, INC.
ANIMAL HEALTH
Shirley, NY 11967
Approved by the FDA under NADA # 140-901
Rev. 09/2021
Carton Labeling
NDC 10797-959-01
Adequani.m.®
polysulfated glycosaminoglycan
PSGAG Injection
100 mg/mL
For intramuscular use in horses only. Not for intra-articular use.
MULTIPLE DOSE VIAL
1 Preserved Vial
[50 mL]
AMERICAN REGENT, INC.
ANIMAL HEALTH
Approved by FDA under NADA # 140-901
Serialization Label
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.