Animal NDC 11695-0094-5 Acepromazine Maleate
Animal Product Information
Acepromazine Maleate Images
Acepromazine Maleate Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
Other
Approved by FDA under NADA # 117-532
For use in dogs only.
Distributed by:
Covetrus North America
400 Metro Drive North
Dublin, OH 43017
covetrus.com
AH-Acepromazine tablets – 02
REV: 0919
670227-04
87017176
Caution:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description:
Acepromazine maleate, a potent neuroleptic agent with a low order of toxicity, is of particular value in the tranquilization of dogs. Its rapid action and lack of hypnotic effect are added advantages.
Chemistry:
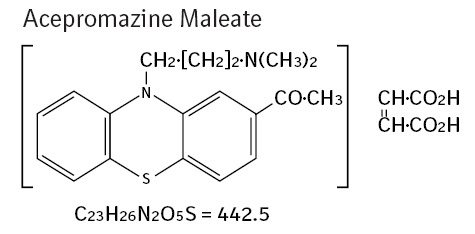
2-acetyl-10-(3-dimethylaminopropyl) phenothiazine hydrogen maleate.
Mode Of Action:
Acepromazine maleate has a depressant effect on the central nervous system and therefore causes sedation, muscular relaxation and a reduction in spontaneous activity. It acts rapidly, exerting a prompt and pronounced calming effect. It is an effective preanesthetic agent and lowers the dosage requirement of general anesthetics.
Indications:
As an aid in tranquilization and as a preanesthetic agent in dogs. Acepromazine maleate tablets can be used as an aid in controlling intractable animals during examination, treatment, grooming, x-ray and minor surgical procedures.
Dosage And Administration:
Dogs: 0.25 - 1.0 mg/lb of body weight. Dosage may be repeated as required.
Animal Safety:
Acute and chronic toxicity studies have shown a very low order of toxicity for acepromazine maleate.
A safety study using elevated dosages of acepromazine maleate demonstrated no adverse reactions even when administered at three times the upper limit of the recommended daily dosage (3.0 mg/lb body weight). The clinical observation for this high dosage was mild depression which disappeared in most dogs 24 hours after termination of dosing.
The only occurrence of adverse reaction during numerous clinical trials was a very mild respiratory distress (reverse sneeze) which was transient in nature and had no effect on the desired action of the drug.
Contraindications:
Phenothiazines may potentiate the toxicity of organophosphates. Therefore, do not use acepromazine maleate to control tremors associated with organic phosphate poisoning.
Do not use in conjunction with organophosphorus vermifuges or ectoparasiticides, including flea collars.
Do not use with procaine hydrochloride.
Warning:
Do not use in animals intended for human consumption.
Precautions:
Tranquilizers are potent central nervous system depressants, and they can cause marked sedation with suppression of the sympathetic nervous system. Tranquilizers can produce prolonged depression or motor restlessness when given in excessive amounts or when given to sensitive animals.
Tranquilizers are additive in action to the actions of other depressants and will potentiate general anesthesia. Tranquilizers should be administered in smaller doses and with greater care during general anesthesia and also to animals exhibiting symptoms of stress, debilitation, cardiac disease, sympathetic blockade, hypovolemia or shock. Acepromazine, like other phenothiazine derivatives, is detoxified in the liver; therefore, it should be used with caution on animals with a previous history of liver dysfunction or leukopenia.
Epinephrine is contraindicated for treatment of acute hypotension produced by phenothiazine-derivative tranquilizers since further depression of blood pressure can occur.
Phenothiazines should be used with caution when followed by epidural anesthetic procedures because they may potentiate the arterial hypotensive effects of local anesthetics.
Adverse Reactions:
A few rare but serious occurrences of idiosyncratic reactions to acepromazine may occur in dogs following oral or parenteral administration. These potentially serious adverse reactions include behavioral disorders in dogs such as aggression, biting/chewing, and nervousness.
To report suspected adverse reactions, to obtain a Safety Data Sheet or for technical assistance, call 1-855-724-3461.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or www.fda.gov/reportanimalae.
Storage:
Store at 20° to 25°C (68° to 77°F), excursions permitted between 15° and 30°C (between 59° and 86°F).
How Supplied:
Acepromazine maleate tablets are available in 10 and 25 mg quarter-scored tablets and are supplied in bottles containing 100 and 500 tablets.
NDC 11695-0093-1 – 10 mg – 100 tablets
NDC 11695-0093-5 – 10 mg – 500 tablets
NDC 11695-0094-1 – 25 mg – 100 tablets
NDC 11695-0094-5 – 25 mg – 500 tablets
Principal Display Panel - Bottle Label, 25 Mg – 500 Tablets
NDC 11695-0094-5
Acepromazine maleate
Tablets 25 mg
CAUTION:Federal law restricts this drug to use by or on the order of a licensed veterinarian.
500 Tablets
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.