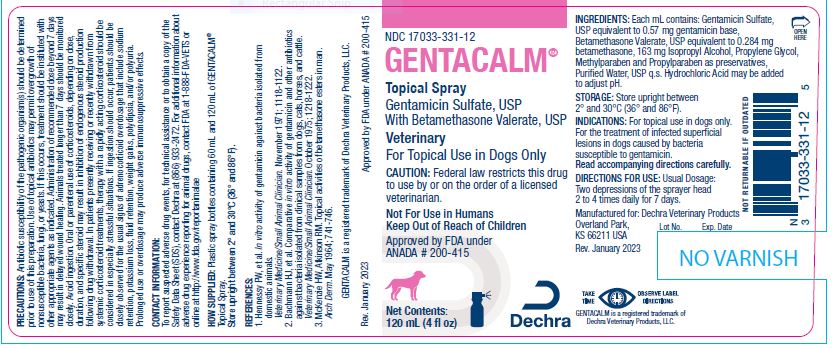
Animal NDC 17033-331-12 Gentacalm
Gentamicin Sulfate
Animal Product Information
Gentacalm Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
- Veterinary Indications
- Caution:
- Ingredients:
- Storage:
- Indications:
- Directions For Use:
- Information For Owners/Caregivers
- Description:
- Chemistry:
- Pharmacology:
- Warning:
- Contraindications:
- Dosage And Administration:
- Toxicity:
- Side Effects:
- Precautions:
- Contact Information:
- How Supplied:
- Storage And Handling
- References:
- Net Contents:60 Ml (2 Fl Oz)
- Net Contents:120 Ml (4 Fl Oz)
Veterinary Indications
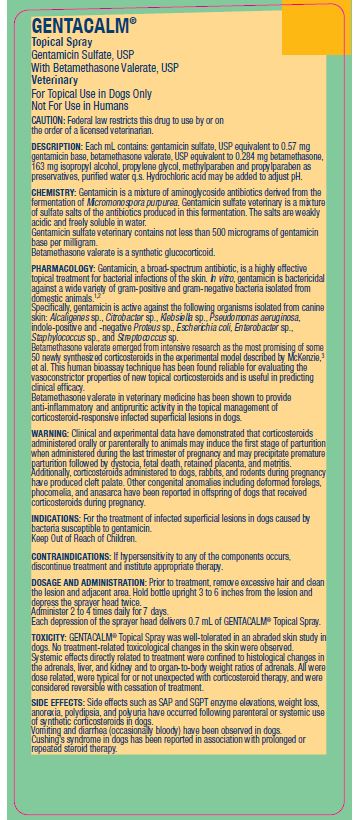
Gentamicin Sulfate, USP With Betamethasone Valerate, USP
Veterinary
For Topical Use in Dogs Only
Caution:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Not For Use in Humans
Keep Out of Reach of Children
Approved by FDA under ANADA # 200-415
Ingredients:
Each mL contains: Gentamicin Sulfate, USP equivalent to 0.57 mg gentamicin base, Betamethasone Valerate, USP equivalent to 0.284 mg betamethasone, 163 mg Isopropyl Alcohol, Propylene Glycol, Methylparaben and Propylparaben as preservatives, Purified Water, USP q.s. Hydrochloric Acid may be added to adjust pH.
Storage:
Store upright between 2° and 30°C (36° and 86°F).
Indications:
For topical use in dogs only. For the treatment of infected superficial lesions in dogs caused by bacteria susceptible to gentamicin.
Read accompanying directions carefully.
For the treatment of infected superficial lesions in dogs caused by bacteria susceptible to gentamicin.
Keep Out of Reach of Children.
Directions For Use:
Usual Dosage: Two depressions of the sprayer head 2 to 4 times daily for 7 days.
Information For Owners/Caregivers
Manufactured for: Dechra Veterinary Products
Overland Park,
KS 66211 USA
Rev. January 2023
GENTACALM is a registered trademark of Dechra Veterinary Products, LLC.
GENTACALM is a registered trademark of Dechra Veterinary Products, LLC.
Rev. January 2023 Approved by FDA under ANADA # 200-415
Description:
Each mL contains: gentamicin sulfate, USP equivalent to 0.57 mg gentamicin base, betamethasone valerate, USP equivalent to 0.284 mg betamethasone, 163 mg isopropyl alcohol, propylene glycol, methylparaben and propylparaben as preservatives, purified water q.s. Hydrochloric acid may be added to adjust pH.
Chemistry:
Gentamicin is a mixture of aminoglycoside antibiotics derived from the fermentation of Micromonospora purpurea. Gentamicin sulfate veterinary is a mixture of sulfate salts of the antibiotics produced in this fermentation. The salts are weakly acidic and freely soluble in water.
Gentamicin sulfate veterinary contains not less than 500 micrograms of gentamicin base per milligram.
Betamethasone valerate is a synthetic glucocorticoid.
Pharmacology:
Gentamicin, a broad-spectrum antibiotic, is a highly effective topical treatment for bacterial infections of the skin. In vitro, gentamicin is bactericidal against a wide variety of gram-positive and gram-negative bacteria isolated from domestic animals.1,2
Specifically, gentamicin is active against the following organisms isolated from canine skin: Alcaligenes sp., Citrobacter sp., Klebsiella sp., Pseudomonas aeruginosa, indole-positive and -negative Proteus sp., Escherichia coli, Enterobacter sp., Staphylococcus sp., and Streptococcus sp.
Betamethasone valerate emerged from intensive research as the most promising of some 50 newly synthesized corticosteroids in the experimental model described by McKenzie,3 et al. This human bioassay technique has been found reliable for evaluating the vasoconstrictor properties of new topical corticosteroids and is useful in predicting clinical efficacy.
Betamethasone valerate in veterinary medicine has been shown to provide anti-inflammatory and antipruritic activity in the topical management of corticosteroid-responsive infected superficial lesions in dogs.
Warning:
Clinical and experimental data have demonstrated that corticosteroids administered orally or parenterally to animals may induce the first stage of parturition when administered during the last trimester of pregnancy and may precipitate premature parturition followed by dystocia, fetal death, retained placenta, and metritis.
Additionally, corticosteroids administered to dogs, rabbits, and rodents during pregnancy have produced cleft palate. Other congenital anomalies including deformed forelegs, phocomelia, and anasarca have been reported in offspring of dogs that received corticosteroids during pregnancy.
Contraindications:
If hypersensitivity to any of the components occurs, discontinue treatment and institute appropriate therapy.
Dosage And Administration:
Prior to treatment, remove excessive hair and clean the lesion and adjacent area. Hold bottle upright 3 to 6 inches from the lesion and
depress the sprayer head twice.
Administer 2 to 4 times daily for 7 days.
Each depression of the sprayer head delivers 0.7 mL of GENTACALM® Topical Spray.
Toxicity:
GENTACALM® Topical Spray was well-tolerated in an abraded skin study in dogs.
No treatment-related toxicological changes in the skin were observed. Systemic effects directly related to treatment were confined to histological changes in the adrenals, liver, and kidney and to organ-to-body weight ratios of adrenals. All were dose related, were typical for or not unexpected with corticosteroid therapy, and were considered reversible with cessation of treatment.
Systemic effects directly related to treatment were confined to histological changes in the adrenals, liver, and kidney and to organ-to-body weight ratios of adrenals. All were dose related, were typical for or not unexpected with corticosteroid therapy, and were considered reversible with cessation of treatment
Side Effects:
Side effects such as SAP and SGPT enzyme elevations, weight loss, anorexia, polydipsia, and polyuria have occurred following parenteral or systemic use of synthetic corticosteroids in dogs.
Vomiting and diarrhea (occasionally bloody) have been observed in dogs. Cushing’s syndrome in dogs has been reported in association with prolonged or repeated steroid therapy.
Precautions:
Antibiotic susceptibility of the pathogenic organism(s) should be determined prior to use of this preparation.
Use of topical antibiotics may permit overgrowth of nonsusceptible bacteria, fungi, or yeasts.
If this occurs, treatment should be instituted with other appropriate agents as indicated.
Administration of recommended dose beyond 7 days may result in delayed wound healing.
Animals treated longer than 7 days should be monitored closely.
Avoid ingestion. Oral or parenteral use of corticosteroids, depending on dose, duration, and specific steroid may result in inhibition of endogenous steroid production following drug withdrawal. In patients presently receiving or recently withdrawn from systemic corticosteroid treatments, therapy with a rapidly acting corticosteroid should be considered in especially stressful situations.
If ingestion should occur, patients should be closely observed for the usual signs of adrenocorticoid overdosage that include sodium retention, potassium loss, fluid retention, weight gains, polydipsia, and/or polyuria. Prolonged use or overdosage may produce adverse immunosuppressive effects.
Contact Information:
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Dechra at (866) 933-2472. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/reportanimalae
How Supplied:
Plastic spray bottles containing 60 mL and 120 mL of GENTACALM® Topical Spray.
Storage And Handling
Store upright between 2° and 30°C (36° and 86°F).
References:
1. Hennessy PW, et al. In vitro activity of gentamicin against bacteria isolated from domestic animals.
Veterinary Medicine/Small Animal Clinician. November 1971; 1118-1122.
2. Bachmann HJ, et al. Comparative in vitro activity of gentamicin and other antibiotics against bacteria isolated from clinical samples from dogs, cats, horses, and cattle.
Veterinary Medicine/Small Animal Clinician. October 1975; 1218-1222.
3. McKenzie HW, Atkinson RM. Topical activities of betamethasone esters in man.
Arch Derm. May 1964; 741-746.
Net Contents:120 Ml (4 Fl Oz)
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.