Animal NDC 23243-2355-1 Tylovet 40 Type A Medicated Article
Tylosin Phosphate
Animal Product Information
| Field Name | Field Value |
|---|---|
| Animal NDC Code | 23243-2355-1 |
| Proprietary Name | Tylovet 40 Type A Medicated Article What is the Proprietary Name? The proprietary name also known as the trade name is the name of the product chosen by the medication labeler for marketing purposes. |
| Non-Proprietary Name | Tylosin Phosphate What is the Non-Proprietary Name? The non-proprietary name is sometimes called the generic name. The generic name usually includes the active ingredient(s) of the product. |
| Labeler Name | Huvepharma, Inc. |
| Product Type | Vfd Type A Medicated Article Animal Drug |
| Usage Information |
|
| Active Ingredient(s) |
|
| Marketing Category | ANADA - ABBREVIATED NEW ANIMAL DRUG APPLICATION What is the Marketing Category? Product types are broken down into several potential Marketing Categories, such as NDA/ANDA/BLA, OTC Monograph, or Unapproved Drug. One and only one Marketing Category may be chosen for a product, not all marketing categories are available to all product types. Currently, only final marketed product categories are included. The complete list of codes and translations can be found at www.fda.gov/edrls under Structured Product Labeling Resources. |
| FDA Application Number | ANADA200484 What is the FDA Application Number? This corresponds to the NDA, ANDA, or BLA number reported by the labeler for products which have the corresponding Marketing Category designated. If the designated Marketing Category is OTC Monograph Final or OTC Monograph Not Final, then the Application number will be the CFR citation corresponding to the appropriate Monograph (e.g. “part 341”). For unapproved drugs, this field will be null. |
Tylovet 40 Type A Medicated Article Images
Tylovet 40 Type A Medicated Article Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
Other
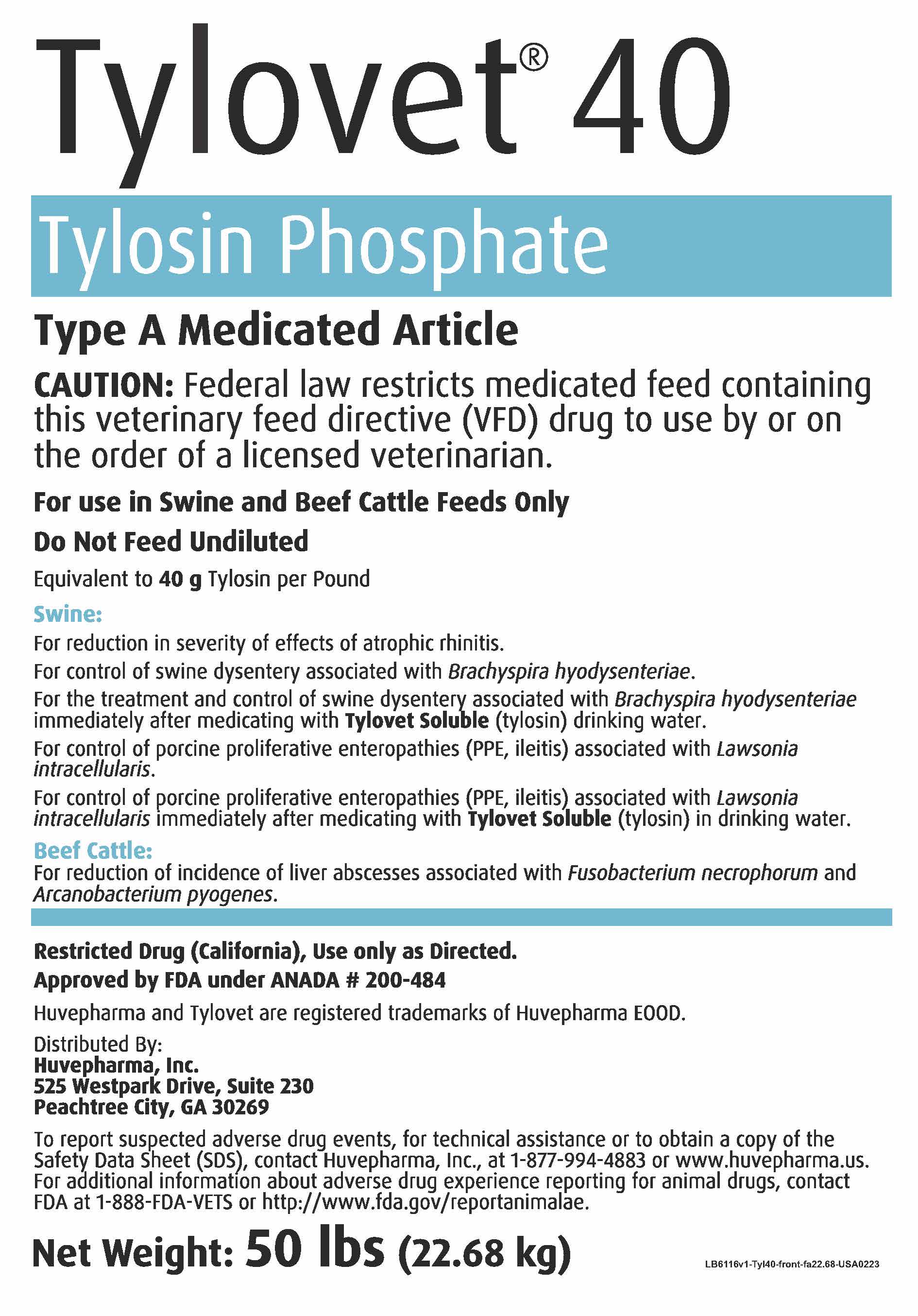
Tylovet® 40
Tylosin Phosphate
Type A Medicated Article
NOT FOR HUMAN USE
Precautions
CAUTION: Federal law restricts medicated feed containing this veterinary feed directive (VFD) drug to use by or on
the order of a licensed veterinarian.
For use in Swine and Beef Cattle Feeds Only
Do Not Feed Undiluted
Indications & Usage
Equivalent to 40 g Tylosin per Pound
Swine:
For the reduction in severity of effects of atrophic rhinitis.
For control of swine dysentery associated with Brachyspira hyodysenteriae.
For the treatment and control of swine dysentery associated with Brachyspira
hyodysenteriae immediately after medicating with Tylovet Soluble (tylosin)
drinking water.
For control of porcine proliferative enteropathies (PPE, ileitis) associated with
Lawsonia intracellularis.
For control of porcine proliferative enteropathies (PPE, ileitis) associated with
Lawsonia intracellularis immediately after medicating with Tylovet Soluble (tylosin)
in drinking water.
Beef Cattle:
For reduction of incidence of liver abscesses associated with Fusobacterium
necrophorum and Arcanobacterium pyogenes.
Directions for Use Read All Directions Carefully
Before Mixing and Feeding
Tylovet® 40 Type A Medicated Article Net Weight: 50 lbs
Do Not Feed Undiluted. (22.68 kgs)
CAUTION: Federal law restricts medicated feed containing this veterinary feed directive (VFD)
drug to use by or on the order of a licensed veterinarian.
Active Drug Ingredient: Tylosin (as tylosin phosphate)...40 g per lb
Ingredients: Roughage products, pregelatanized starch and dipotassium phosphate.
Caution: To ensure adequate mixing, an intermediate blending step should be used prior to
manufacturing a complete feed. Do not use in any liquid feed containing sodium metabisulfite
or in any finished feed (supplement, concentrate or complete feed) containing in excess of 2%
bentonite.
Warning:Tylovet 40 may be irritating to unprotected skin and eyes. When mixing and handling
Tylovet 40 use protective clothing, impervious gloves and a dust respirator. In case of
accidental eye exposure, flush eyes with plenty of water. Exposed skin should be washed with plenty
of soap and water. Remove and wash contaminated clothing. Seek medical attention if irritation
becomes severe or persists. The Safety Data Sheet (SDS) contains more detailed occupational
safety information. To report adverse effects, access medical information, or obtain additional
product information, call 1-877-994-4883.
For reduction in severity of effects of atrophic rhinitis. Feed 100 g of tylosin per ton
(2.5 pounds Tylovet 40 per ton) of complete feed. Feed continuously as the sole ration.
For control of swine dysentery. Feed 100 g of tylosin per ton (2.5 pounds Tylovet 40 per ton)
of complete feed for at least three weeks. Follow with 40 g tylosin per ton (1.0 pound
Tylovet 40 per ton) of complete feed until pigs reach market weight.
For the treatment and control of swine dysentery. Feed 40 to 100 grams of tylosin (1.0 to
2.5 pounds of Tylovet 40) per ton of complete feed for 2 to 6 weeks immediately after
medicating with 250 mg tylosin (as Tylovet Soluble) per gallon in drinking water for 3 to 10
days.
For the control of porcine proliferative enteropathies (PPE, ileitis). Feed 100 g tylosin
per ton (2.5 pound Tylovet 40 per ton) of complete feed for 21 days. Alternatively, feed 100 g
of tylosin per ton (2.5 pound Tylovet 40 per ton) of complete feed for at least three weeks,
followed by 40 g tylosin per ton of complete feed until pigs reach market weight. Alternatively,
feed 40 to 100 grams of tylosin (1.0 to 2.5 pounds of Tylovet 40) per ton of complete feed for 2
to 6 weeks immediately after medicating with 250 mg tylosin (as Tylovet Soluble) per gallon in
drinking water for 3 to 10 days. Feed continuously as the sole ration when feeding Tylovet.
NOTICE: Organisms vary in their degree of susceptibility to any chemotherapeutic. If no
improvement is observed after recommended treatment, diagnosis and susceptibility should be
reconfirmed.
Mixing and Feeding | Tylovet 40 per Ton | Tylosin Per Ton of |
0.2 to 0.25 lbs
| 8 to 10 g | |
Mixing Directions | LIMITATIONS: | |
General Precautions
Restricted Drug (California) Use Only as Directed.
Approved by FDA under ANADA # 200-484
Huvepharma and Tylovet are registered trademarks of Huvepharma EOOD.
Distributed By:
Huvepharma, Inc.
525 Westpark Drive, Suite 230
Peachtree City, GA 30269
Adverse Reactions
To report suspected adverse drug events, for technical assistance or to obtain
a copy of the Safety Data Sheet (SDS), contact Huvepharma, Inc., at
1-877-994-4883 or www.huvepharma.us. For additional information about adverse
drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or
http://www.fda.gov/reportanimalae.
Net Weight: 50 lbs (22.68 kg)
Storage And Handling
Store at Room Temperature, 20 - 25°C (68 - 77°F). Avoid moisture.
Not to be used after the date printed on the bottom of the bag.
Rev. 06-2021
Package Label.Principal Display Panel
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.