Animal NDC 23243-7287-1 Tilmovet Ac Oral Solution
Tilmicosin Phosphate
Animal Product Information
| Field Name | Field Value |
|---|---|
| Animal NDC Code | 23243-7287-1 |
| Proprietary Name | Tilmovet Ac Oral Solution What is the Proprietary Name? The proprietary name also known as the trade name is the name of the product chosen by the medication labeler for marketing purposes. |
| Non-Proprietary Name | Tilmicosin Phosphate What is the Non-Proprietary Name? The non-proprietary name is sometimes called the generic name. The generic name usually includes the active ingredient(s) of the product. |
| Labeler Name | Huvepharma, Inc. |
| Product Type | Prescription Animal Drug |
| Usage Information |
|
| Active Ingredient(s) |
|
| Marketing Category | ANADA - ABBREVIATED NEW ANIMAL DRUG APPLICATION What is the Marketing Category? Product types are broken down into several potential Marketing Categories, such as NDA/ANDA/BLA, OTC Monograph, or Unapproved Drug. One and only one Marketing Category may be chosen for a product, not all marketing categories are available to all product types. Currently, only final marketed product categories are included. The complete list of codes and translations can be found at www.fda.gov/edrls under Structured Product Labeling Resources. |
| FDA Application Number | ANADA200707 What is the FDA Application Number? This corresponds to the NDA, ANDA, or BLA number reported by the labeler for products which have the corresponding Marketing Category designated. If the designated Marketing Category is OTC Monograph Final or OTC Monograph Not Final, then the Application number will be the CFR citation corresponding to the appropriate Monograph (e.g. “part 341”). For unapproved drugs, this field will be null. |
Tilmovet Ac Oral Solution Images
Tilmovet Ac Oral Solution Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
- Front Panel
- Warning
- Caution:
- Indications:
- Dosage And Administration:
- Warnings:User Safety Warnings:
- Residue Warning:
- Description:
- Note To The Physician:
- Precautions:
- Adverse Reactions In Animals:
- Clinical Pharmacology:
- Effectiveness:
- Animal Safety:
- How Supplied:
- Storage Conditions:
- Advertencia
- Precauclón:
- Lndicacions:
- Posologia Y Administración:
- Advertencias:Advertencias De Seguridad Para El Usuario:
- Advertencia Acerca De Residuos:
- Descripción:
- Indicacions:
- Posología Y Administración:
- Nota Para El Médico:
- Precauciones:
- Reacciones Adversas En Animales:
- Presentación:
- Condiciones De Almacenamiento:
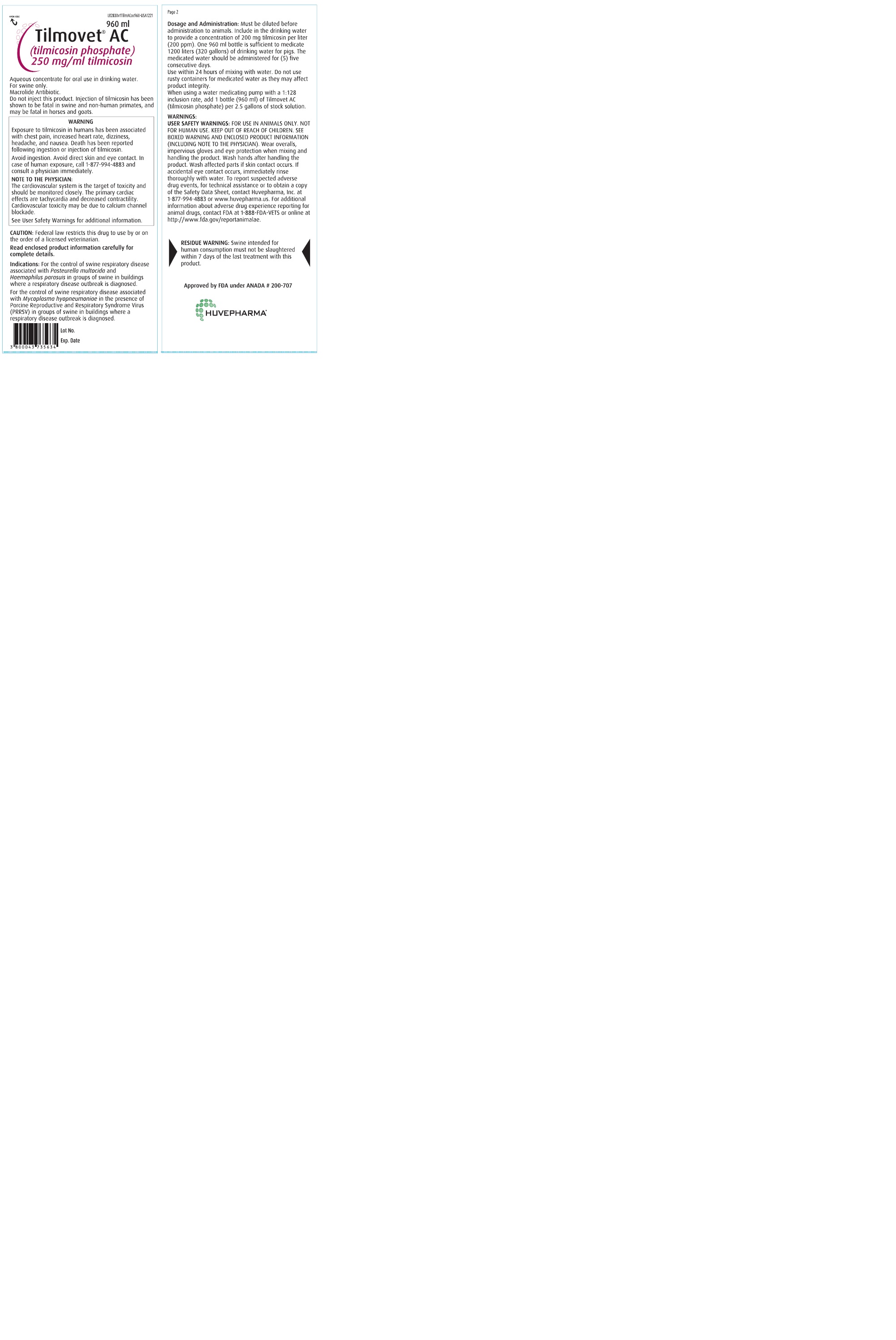
Front Panel
960 ml
Tilmovet® AC
(tilmicosin phosphate)
250 mg/ml tilmicosin
Aqueous concentrate for oral use in drinking water.
For swine only.
Macrolide Antibiotic.
Do not inject this product. Injection of tilmicosin has been shown to be fatal in swine and non-human primates, and may be fatal in horses and goats.
960 ml
Tilmovet® AC
tilmicosina como fosfato
(250 mg/ml tilmicosina)
Concentrado acuoso para uso oral en el agua de consumo.
Para ganado porcine únicamente.
Antibiótico macrólido.
No inyecte este producto. Se demostró que la inyección
de tilmicosina es fatal para el ganado porcino y en
primates no humanos, y podría ser fatal para caballos y
cabras.
Warning
Exposure to tilmicosin in humans has been associated with chest pain, increased heart rate, dizziness,
headache, and nausea. Death has been reported following ingestion or injection of tilmicosin.
Avoid ingestion. Avoid direct skin and eye contact. In case of human exposure, call 1-877-994-4883 and
consult a physician immediately.
NOTE TO THE PHYSICIAN:
The cardiovascular system is the target of toxicity and should be monitored closely. The primary cardiac
effects are tachycardia and decreased contractility. Cardiovascular toxicity may be due to calcium channel
blockade.
See User Safety Warnings for additional information.
Exposure to tilmicosin in humans has been associated with chest pain, increased heart rate, dizziness,
headache, and nausea. Death has been reported following ingestion or injection of tilmicosin.
Avoid ingestion. Avoid direct skin and eye contact. In case of human exposure, call 1-877-994-4883 and
consult a physician immediately.
NOTE TO THE PHYSICIAN:
The cardiovascular system is the target of toxicity and should be monitored closely. The primary cardiac
effects are tachycardia and decreased contractility. Cardiovascular toxicity may be due to calcium channel
blockade.
See User Safety Warnings for additional information.
Caution:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Read enclosed product information carefully for complete details.
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Active Drug Ingredient: tilmicosin (as tilmicosin phosphate) 250 mg/ml
Indications:
For the control of swine respiratory disease associated with Pasteurella multocida and
Haemophilus parasuis in groups of swine in buildings where a respiratory disease outbreak is diagnosed.
For the control of swine respiratory disease associated with Mycoplasma hyopneumoniae in the presence of
Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) in groups of swine in buildings where a
respiratory disease outbreak is diagnosed.
Lot No.
Exp. Date
For the control of swine respiratory disease associated with Pasteurella multocida and Haemophilus parasuis
in groups of swine in buildings where a respiratory disease outbreak is diagnosed.
For the control of swine respiratory disease associated with Mycoplasma hyopneumoniae in the presence of
Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) in groups of swine in buildings where a
respiratory disease outbreak is diagnosed.
Dosage And Administration:
Must be diluted before administration to animals. Include in the drinking water to provide a concentration of
200 mg tilmicosin per liter (200 ppm). One 960 ml bottle is sufficient to medicate 1200 liters (320 gallons)
of drinking water for pigs. The medicated water should be administered for (5) five consecutive days.
Use within 24 hours of mixing with water. Do not use rusty containers for medicated water as they may affect
product integrity.
When using a water medicating pump with a 1:128 inclusion rate, add 1 bottle (960 ml) of Tilmovet AC
(tilmicosin phosphate) per 2.5 gallons of stock solution.
Must be diluted before administration to animals. Include in the drinking water to provide a concentration of
200 mg tilmicosin per liter (200 ppm). One 960 ml bottle is sufficient to medicate 1200 liters (320 gallons)
of drinking water for pigs. The medicated water should be administered for (5) five consecutive days.
Use within 24 hours of mixing with water. Do not use rusty containers for medicated water as they may affect
product integrity.
When using a water medicating pump with a 1:128 inclusion rate, add 1 bottle (960 ml) of Tilmovet AC
(tilmicosin phosphate) per 2.5 gallons of stock solution.
Warnings:User Safety Warnings:
FOR USE IN ANIMALS ONLY. NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN. SEE
BOXED WARNING AND ENCLOSED PRODUCT INFORMATION (INCLUDING NOTE TO THE PHYSICIAN).
Wear overalls, impervious gloves and eye protection when mixing and handling the product. Wash
hands after handling the product. Wash affected parts if skin contact occurs. If accidental eye
contact occurs, immediately rinse thoroughly with water. To report suspected adverse drug events,
for technical assistance or to obtain a copy of the Safety Data Sheet, contact Huvepharma, Inc. at
1-877-994-4883 or www.huvepharma.us. For additional information about adverse drug experience
reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/reportanimalae.
FOR USE IN ANIMALS ONLY. NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN. SEE
BOXED WARNING AND ENCLOSED PRODUCT INFORMATION (INCLUDING NOTE TO THE PHYSICIAN).
Wear overalls, impervious gloves and eye protection when mixing and handling the product. Wash
hands after handling the product. Wash affected parts if skin contact occurs. If accidental eye
contact occurs, immediately rinse thoroughly with water. To report suspected adverse drug events,
for technical assistance or to obtain a copy of the Safety Data Sheet, contact Huvepharma, Inc. at
1-877-994-4883 or www.huvepharma.us. For additional information about adverse drug experience
reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/reportanimalae.
Residue Warning:
Swine intended for human consumption must not be slaughtered within 7 days of the last treatment with this
product.
Approved by FDA under ANADA # 200-707
Swine intended for human consumption must not be slaughtered within 7 days of the last treatment with this
product.
Description:
Tilmovet AC is a formulation of the antibiotic tilmicosin. Tilmicosin is produced semisynthetically
and is in the macrolide class of antibiotics. Each milliliter (ml) of Tilmovet aqueous concentrate
solution contains 250 mg of tilmicosin.
Note To The Physician:
The cardiovascular system is the target of toxicity and should be monitored closely. Cardiovascular toxicity
may be due to calcium channel blockade. In dogs, administration of intravenous calcium offset tilmicosin-induced
tachycardia and negative inotropy (decreased contractility). Dobutamine partially offset the negative inotropic
effects induced by tilmicosin injection in dogs. β-adrenergic antagonists, such as propranolol, exacerbated the
negative inotropy of tilmicosin injection in dogs. Epinephrine potentiated lethality of tilmicosin injection in pigs.
This antibiotic persists in tissues for several days.
Precautions:
Do not allow horses or other equines access to water containing tilmicosin. The safety of tilmicosin
has not been established in male swine intended for breeding purposes.
Always treat the fewest number of animals necessary to control a respiratory disease outbreak. Prescriptions
shall not be refilled. Concurrent use of Tilmovet AC (tilmicosin phosphate) and another macrolide by any
route is not advised. Use of another macrolide immediately following this use of Tilmovet AC is not advised.
Adverse Reactions In Animals:
Decreased water consumption was observed in healthy pigs administered tilmicosin in target animal safety studies.
Ensure that pigs have continuous access to medicated water during the treatment period. Monitor pigs for signs of
water refusal and dehydration while being treated. If decreased water consumption occurs, replace the medicated
drinking water with fresh nonmedicated water and contact your veterinarian.
Clinical Pharmacology:
Tilmicosin is a macrolide antibiotic with in vitro antibacterial activity primarily against Gram-positive
bacteria, although certain Gram-negative bacteria are also susceptible. Macrolides interfere with
bacterial protein synthesis by reversibly binding to the 50S subunit of the ribosome. They are
typically regarded as being bacteriostatic, but at high concentrations can be bactericidal.
When administered orally to pigs via the drinking water, tilmicosin is rapidly absorbed and slowly
eliminated from the body. Tilmicosin distributes rapidly to the target tissues. Detectable levels
are found in lung tissue as early as 6 hours and peak at about 5 days after the commencement of
treatment. The relationship of serum tilmicosin concentration to lung tilmicosin concentration or the
concentrations in bronchial secretion has not been determined. In addition, the extent to which total lung
concentrations represent free (active) drug has not been defined. Therefore, no conclusions can be
made with regard to the clinical relevance of elevated tilmicosin concentrations in the lung. Tilmicosin
has been shown to concentrate within alveolar macrophages. It is also found at fairly high concentrations
in liver and kidney tissue, as it is excreted both via the bile into the feces and also via the urine.
Effectiveness:
The effectiveness of tilmicosin phosphate for the control of SRD associated with P. multocida and H. parasuis was
confirmed in a natural infection field study across six U.S. sites. A total of 960 commercial-type grower pigs were
enrolled and assigned to the tilmicosin-treated group (200 mg tilmicosin/L in drinking water for 5 consecutive days),
or a non-medicated control group.
Pigs that 1) were found dead and were diagnosed with SRD, or 2) had a depression score and a respiratory score ≥ 2
(on a scale from 0 [normal] to 3 [severe]) and a rectal temperature of ≥ 104.5 °F were considered clinically affected.
At each site, treatments were initiated when at least 15% of the pigs were classified as clinically affected. After
the 5-day treatment period and a 4-day post-treatment period, pigs were evaluated for treatment success (respiration
and depression scores of 1 or 0 and rectal temperature < 104.5 °F), and were euthanized and evaluated for lung lesions.
A significantly higher (p = 0.0118)success rate (based on back-transformed least squares means) was detected for the
tilmicosin-treated group (275/473, 58.64%) compared to the control group (230/475, 47.89%).
The effectiveness of tilmicosin phosphate for the control of SRD associated with M. hyopneumoniae in the presence of
PRRSV was confirmed in an induced infection model study. A total of 340 commercial-type pigs were enrolled and challenged
with M. hyopneumoniae (single infection) or M. hyopneumoniae and PRRSV (co-infection). When necropsied sentinel pigs had
at least 5% lung lesion involvement, study pigs were treated with tilmicosin phosphate (200 mg tilmicosin/L in drinking
water) or non-medicated water for 5 consecutive days. After the 5 day treatment period and a 4 day post-treatment period,
pigs were euthanized and evaluated for lung lesions.
For both the single infection and co-infection groups, the lung lesion percentage was statistically significantly different
(p=0.005 and p=0.0004, respectively) in favor of the tilmicosin phosphate-treated group (21.01% and 31.74%, respectively)
compared with the control group (28.26% and 43.04%, respectively).
Animal Safety:
A pharmacokinetic study was conducted to evaluate tilmicosin phosphate solution in pigs. The results
were compared to pharmacokinetic data generated with tilmicosin phosphate Type A medicated article
(NADA 141-064). The data demonstrates that blood and tissue levels of tilmicosin when administered to
pigs at 200 mg/L (ppm) in water were consistently lower than when tilmicosin was administered to pigs at
181 g/ton (200 ppm) in feed.
A target animal safety study was conducted to evaluate the tolerance of tilmicosin phosphate solution in
pigs when administered in drinking water.
Twenty pigs were administered medicated water at 0, 200, 400, or 600 mg/ L (0, 1X, 2X, or 3X the labeled
dose) for 5 consecutive days or 200 mg/ L for 10 consecutive days. No treatment-related lesions were
observed in any animals at necropsy. Water consumption was decreased in all tilmicosin-treated groups
compared to the non-medicated group. One pig in the 600 mg/ L group was euthanized due to decreased water
consumption, neurological signs attributed to severe dehydration, and subsequent refusal to drink
non-medicated water. Two pigs in the 400 mg/ L group had reduced water intake and displayed mild clinical
signs attributed to dehydration. One pig recovered after being offered non-medicated water. The second pig
completed the treatment regimen without intervention.
Hydration and water consumption were evaluated during the control of SRD effectiveness field study.
Tilmicosin was administered to study pigs in drinking water at 200 mg/l for 5 consecutive days. There was
no statistically significant difference in water consumption between tilmicosin-treated pigs and pigs
receiving nonmedicated water. A subset of study pigs (20 tilmicosin treated pigs and 20 non-medicated pigs)
were evaluated for hydration via a physical examination and analysis of blood samples for hematocrit, total
protein, creatinine, and blood urea nitrogen. There were no abnormal physical examination findings or
clinically relevant differences in clinical pathology variables between tilmicosin-treated pigs and pigs
receiving nonmedicated water.
How Supplied:
Tilmovet AC (tilmicosin phosphate) is provided in a 960 ml white-colored plastic bottle sealed with a plastic screw cap.
Storage Conditions:
Store at or below 25° C (77° F). Excursions permitted to 40° C (104° F). Protect from direct sunlight.
Restricted Drug (California) - Use Only as Directed
Approved by FDA under ANADA # 200-707
Manufactured For:
Huvepharma, Inc.
525 Westpark Drive, Suite 230
Peachtree City, GA 30269
Huvepharma and Tilmovet are registered trademarks of
Huvepharma EOOD.
Advertencia
La exposición humana a la tilmicosina ha sido asociada con dolor en el pecho, aumenta de la
frecuencia cardíaca, mareo, dolor de cabeza y náuseas. Se ha informado de fallecimientos tras
la ingestión o inyección de la tilmicosina.
Evite su ingestión. Evite el contacto directo con la piel y los ojos. En caso de que ocurra alguna
exposición humana, llame al 1-877-994-4883 y consulte inmediatamente con el médico.
NOTA PARA EL MÉDICO:
El sistema cardiovascular es el objetivo de la toxicidad y debe controlarse atentamente. Los
efectos cardíacos principales son taquicardia y una disminución de la contractilidad. La
toxicidad cardiovascular puede deberse al bloqueo de los canales de calcio.
Examine las Advertencias de seguridad para el usuario para obtener informacin adicional.
La exposición humana a la tilmicosina ha sido asociada con dolor en el pecho, aumenta de la
frecuencia cardíaca, mareo, dolor de cabeza y náuseas. Se ha informado de fallecimientos tras
la ingestión o inyección de la tilmicosina.
Evite su ingestión. Evite el contacto directo con la piel y los ojos. En caso de que ocurra alguna
exposición humana, llame al 1-877-994-4883 y consulte inmediatamente con el médico.
NOTA PARA EL MÉDICO:
El sistema cardiovascular es el objetivo de la toxicidad y debe controlarse atentamente. Los
efectos cardíacos principales son taquicardia y una disminución de la contractilidad. La
toxicidad cardiovascular puede deberse al bloqueo de los canales de calcio. Examine las
Advertencias de seguridad para el usuario para obtener informacin adicional.
Precauclón:
las leyes federales establecen que el uso de este fármaco se restrinja a veterinarios con licencia
o bajo la indicación de estos.
Lea cuidadosamente la información del producto que se adjunta para conocer los detalles completos.
las leyes federales establecen que el uso de este fármaco se restrinja a veterinarios con licencia
o bajo la indicación de estos.
Ingrediente fármaco activo: tilmicosina (como fosfato de tilmicosina) 250 mg/ml
Lndicacions:
para el control de la enfermedad respiratoria porcina asociada con Pasteurella multocida y Haemophilus
parasuis en grupos de cerdos de establecimientos en las que se diagnostica un brote de enfermedad
respiratoria.
Para el control de la enfermedad respiratoria porcina asociada con Mycoplosma hyopneumoniae
en la presencia del virus del sindrome reproductivo y respiratorio porcino (Porcine Reproductive and
Respiratory Syndrome Virus, PRRSV) en grupos de cerdos en edificios donde se diagnostica un brote de
enfermedad respiratoria.
Lot:
Exp.
Posologia Y Administración:
se debe diluir antes de administrarse a los animales. lncluir en el agua de consumo
para proporcionar una concentración de 200 mg de tilmicosina por litro (200 ppm). Un
frasco de 960 ml es suficiente para medicar 1200 litros (320 galones) de agua de
consumo para cerdos. El agua medicada debería administrarse durante 5 (cinco) días
consecutivos. Usar dentro de las 24 horas de mezclarlo con agua. No use recipientes
oxidados para el agua medicada, ya que los mismos podrían afectar la integridad del
producto.
Si se usa una bomba de medicación de agua con una tasa de inclusión de 1:128,
agregar 1 frasco (960 ml) de Tilmovet AC (tilmicosina coma fosfato) por cada 2.5 galones
de solucion concentrada.
Advertencias:Advertencias De Seguridad Para El Usuario:
PARA SU USO EN ANIMALES ÚNICAMENTE. ESTE PRODUCTO NO DEBE UTILIZARSE
EN SERES HUMANOS. MANTENGAFUERA DEL ALCANCE DE LOS NIÑOS. EXAMINE LA
ADVERTENCIA PRESENTE EN LA CAJA Y LA INFORMAClÓN DEL PRODUCTO QUE SE
ADJUNTA (INCLUIDA LA NOTA PARA EL MÉDICO). Use monos de trabajo, guantes
impermeables y protección ocular al mezclar y manipular el producto.
Lávese las manos después de manipular el producto. Lave las partes
afectadas en caso de producirse el contacto con la piel. Si se produce
contacto accidental con los ojos, inmediatamente enjuague bien con agua.
Para informar sospechas de eventos adversos del fármaco, recibir asistencia técnica u
obtener una copia de laobtener asistencia ténica o una hoja de datos de seguridad
(Safety Data Sheet, SDS), comuníquese con Huvepharma, Inc. al 1-877-994-4883
o www.huvepharma.us. Para obtener información adicional sobre la experiencia
farmacológica adversa para la presentación de informes de fármacos para animales,
comuníquese con la FDA al 1-888-FDA-VETS, o en línea en www.fda.gov/reportanimalae.
PARA SU USO EN ANIMALES ÚNICAMENTE. ESTE PRODUCTO NO DEBE UTILIZARSE
EN SERES HUMANOS. MANTENGAFUERA DEL ALCANCE DE LOS NIÑOS. EXAMINE LA
ADVERTENCIA PRESENTE EN LA CAJA Y LA INFORMAClÓN DEL PRODUCTO QUE SE
ADJUNTA (INCLUIDA LA NOTA PARA EL MÉDICO). Use monos de trabajo, guantes
impermeables y protección ocular al mezclar y manipular el producto.
Lávese las manos después de manipular el producto. Lave las partes
afectadas en caso de producirse el contacto con la piel. Si se produce
contacto accidental con los ojos, inmediatamente enjuague bien con agua.
Para informar sospechas de eventos adversos del fármaco, recibir asistencia técnica u
obtener una copia de laobtener asistencia ténica o una hoja de datos de seguridad
(Safety Data Sheet, SDS), comuníquese con Huvepharma, Inc. al 1-877-994-4883
o www.huvepharma.us. Para obtener información adicional sobre la experiencia
farmacológica adversa para la presentación de informes de fármacos para animales,
comuníquese con la FDA al 1-888-FDA-VETS, o en línea en www.fda.gov/reportanimalae.
Advertencia Acerca De Residuos:
los cerdos para consumo humano no se deberán faenar dentro de los 7 días posteriores
al último tratamiento con este producto.
Aprobado por la FDA bajo ANADA # 200-707
los cerdos para consumo humano no se deberán faenar dentro de los 7 días posteriores
al último tratamiento con este producto.
Descripción:
Tilmovet AC es una formulación del antibiótico tilmicosina. La tilmicosina se produce en forma
semisintética y pertenece a la clase de antibióticos macrólidos. Cada mililitro (ml) de solución
de concentrado acuoso Tilmovet contiene 250 mg de tilmicosina.
Indicacions:
para el control de la enfermedad respiratoria porcina asociada con Pasteurella multocida y
Haemophilus parasuis en grupos de cerdos de establecimientos en los que se diagnostica
un brote de enfermedad respiratoria.
Para el control de la enfermedad respiratoria porcina asociada con Mycoplasma hyopneumoniae en la
presencia del virus del síndrome reproductivo y respiratorio porcino (Porcine Reproductive and
Respiratory Syndrome Virus, PRRSV) en grupos de cerdos en edificios donde se diagnostica un brote de
enfermedad respiratoria.
Posología Y Administración:
se debe diluir antes de administrarse a los animales. lncluir en el agua de consumo
para proporcionar una concentración de 200 mg de tilmicosina por litro (200 ppm). Un
frasco de 960 ml es suficiente para medicar 1200 litros (320 galones) de agua de
consumo para cerdos. El agua medicada debería administrarse durante 5 (cinco) días
consecutivos. Usar dentro de las 24 horas de mezclarlo con agua. No use recipientes
oxidados para el agua medicada, ya que las mismos podrían afectar la integridad del
producto.
Si se usa una bomba de medicación de agua con una tasa de inclusión de 1:128,
agregar 1 frasco (960 ml) de Tilmovet AC (tilmicosina coma fosfato) par cada 2.5 galones
de solucin concentrada.
Nota Para El Médico:
El sistema cardiovascular es el objetivo de la toxicidad y debe controlarse
atentamente. La toxicidad cardiovascular puede deberse al bloqueo de las
canales de calcio. En los perros, la administración de calcio por vía
intravenosa contrarresta la taquicardia inducida por la tilmicosina y el
inotropismo negativo (disminución de la contractilidad). La dobutamina
contrarresta parcialmente los efectos inotrópicos negatives inducidos por
la tilmicosina en perros. Los antagonistas β adrenérgicos, como el propranolol,
exacerbaron el inotropismo negative de la inyección de tilmicosina en perros.
La epinefrina potenció la letalidad de la inyección de tilmicosina en cerdos.
Este antibiótico persiste en los tejidos durante varios días.
Precauciones:
No permita que caballos u otros equinos accedan al agua que contiene tilmicosina.
No se ha establecido la seguridad de la tilmicosina en cerdos machos que se usan
con fines de reproducción.
Trate siempre el menor número de animales necesario para controlar un brote de
enfermedad respiratoria. Las recetas no deben renovarse.
No se recomienda el uso simultáneo de Tilmovet AC (tilmicosina coma fosfato)
y otro macrólido por cualquier vía de administración. No se recomienda el uso de
otro macrólido inmediatamente después de este uso de Tilmovet AC.
Reacciones Adversas En Animales:
se observó una disminución en el consumo de agua en cerdos saludables
a los que se administró tilmicosina en estudios de seguridad en animales
objetivo. Asegúrese de que los cerdos tengan acceso constante al agua
medicada durante el período de tratamiento. Controle que las cerdos no
presenten signos de rechazo al agua y deshidratación mientras se las esté
tratando. Si se produce una disminución en el consumo de agua, reemplace
el agua de consumo medicada por agua potable sin medicar y comuníquese
con su veterinario.
Presentación:
Tilmovet AC se proporciana en botellas de plástico, color blanco de 960 ml selladas con tapas
plásticas con rosca.
Condiciones De Almacenamiento:
Almacenar a o menos 25° C (77° F). Excursiones permitidas a 40° C (104° F).
Proteger de la luz solar directa.
Fármaco restringido (California). Usar únicamente según
las indicaciones.
Aprobado por la FDA bajo ANADA # 200-707
Fabricado por:
Huvepharma, Inc.
525 Westpark Drive, Suite 230
Peachtree City, GA 30269, EE. UU.
Huvepharma y Tilmovet son marcas registradas propiedad
a Huvepharma EOOD.
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.