Animal NDC 46066-022-05 Bactracillin G
Penicillin G Procaine
Animal Product Information
| Field Name | Field Value |
|---|---|
| Animal NDC Code | 46066-022-05 |
| Proprietary Name | Bactracillin G What is the Proprietary Name? The proprietary name also known as the trade name is the name of the product chosen by the medication labeler for marketing purposes. |
| Non-Proprietary Name | Penicillin G Procaine What is the Non-Proprietary Name? The non-proprietary name is sometimes called the generic name. The generic name usually includes the active ingredient(s) of the product. |
| Labeler Name | Aspen Veterinary Resources |
| Product Type | Prescription Animal Drug |
| Usage Information |
|
| Active Ingredient(s) |
|
| Inactive Ingredient(s) |
|
| Marketing Category | NADA - NEW ANIMAL DRUG APPLICATION What is the Marketing Category? Product types are broken down into several potential Marketing Categories, such as NDA/ANDA/BLA, OTC Monograph, or Unapproved Drug. One and only one Marketing Category may be chosen for a product, not all marketing categories are available to all product types. Currently, only final marketed product categories are included. The complete list of codes and translations can be found at www.fda.gov/edrls under Structured Product Labeling Resources. |
| FDA Application Number | NADA065010 What is the FDA Application Number? This corresponds to the NDA, ANDA, or BLA number reported by the labeler for products which have the corresponding Marketing Category designated. If the designated Marketing Category is OTC Monograph Final or OTC Monograph Not Final, then the Application number will be the CFR citation corresponding to the appropriate Monograph (e.g. “part 341”). For unapproved drugs, this field will be null. |
Bactracillin G Images
Bactracillin G Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
Other
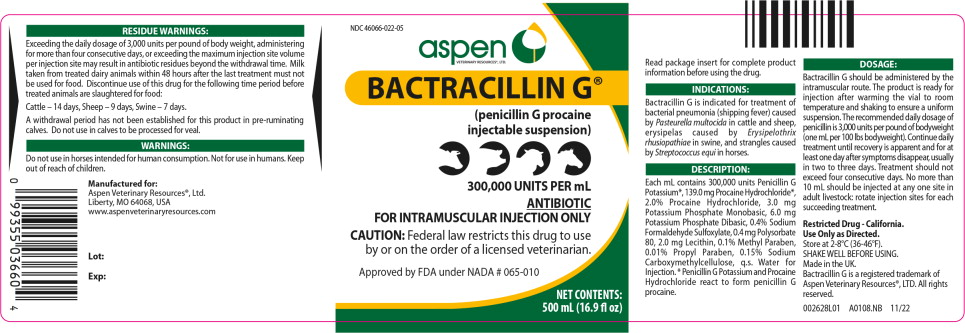
Approved by FDA under NADA # 065-010
BACTRACILLIN G®
(penicillin G procaine injectable suspension)
For use in Cattle, Sheep, Swine and Horses.
ANTIBIOTIC
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
READ ENTIRE BROCHURE CAREFULLY BEFORE USING THIS PRODUCT
Description:
Bactracillin G is a suspension of penicillin G procaine in 100, 250, and 500 mL multiple dose vials. Each mL is designed to provide 300,000 units of penicillin G as procaine in a stable suspension. Penicillin G procaine is an antibacterial agent which has activity against a variety of pathogenic organisms, mainly in the Gram-positive category.
Indications:
Bactracillin G is indicated for treatment of bacterial pneumonia (shipping fever) caused by Pasteurella multocida in cattle and sheep, erysipelas caused by Erysipelothrix rhusiopathiae in swine, and strangles caused by Streptococcus equi in horses.
Directions For Use:
A thoroughly cleaned, sterile needle and syringe should be used for each injection (needles and syringes may be sterilized in boiling water for 15 minutes). Before withdrawing the solution from the bottle, disinfect the rubber cap top with 70% alcohol. The injection site should be similarly disinfected with alcohol. Needles of 16 to 18 gauge and 1 to 1.5 inches long are adequate for intramuscular injections.
In livestock intramuscular injections should be made by directing the needle of suitable gauge and length into the fleshy part of a thick muscle, such as rump, hip, or thigh region; avoid blood vessels and major nerves. Before injecting the solution, pull back gently on the plunger. If blood appears in the syringe, a blood vessel has been entered; withdraw the needle and select a different site.
Dosage:
Bactracillin G is administered by the intramuscular route. The product is ready for injection after warming the vial to room temperature and shaking to ensure a uniform suspension.
The daily dose of penicillin is 3,000 units per pound of body weight (1 mL per 100 lbs body weight). Continue daily treatment until recovery is apparent and for at least one day after symptoms disappear, usually in two to three days.
Treatment should not exceed four consecutive days.
No more than 10 mL should be injected at any one site. Rotate injection sites for each succeeding treatment.
Care Of Sick Animals:
The use of antibiotics in the management of diseases is based on an accurate diagnosis and an adequate course of treatment. When properly used in the treatment of diseases caused by penicillin-susceptible organisms, most animals treated with Bactracillin G show a noticeable improvement within 24 to 48 hours. If improvement does not occur within this period of time, the diagnosis and course of treatment should be re-evaluated. It is recommended that the diagnosis and treatment of animal diseases be carried out by a veterinarian.
Since many diseases look alike but require different types of treatment, the use of professional veterinary and laboratory services can reduce treatment time, costs and needless losses. Good housing, sanitation and nutrition are important in the maintenance of healthy animals and are essential in the treatment of disease.
Residue Warnings:
Exceeding the daily dosage of 3,000 units per pound of body weight, administering for more than four consecutive days, or exceeding the maximum injection site volume per injection site may result in antibiotic residues beyond the withdrawal time. Milk taken from treated dairy animals within 48 hours after the last treatment must not be used for food. Discontinue use of this drug for the following time period before treated animals are slaughtered for food:
Cattle – 14 days, Sheep – 9 days,
Swine – 7 days.
A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in calves to be processed for veal.
Warning:
Do not use in horses intended for human consumption. Not for use in humans.
Keep out of reach of children.
Precautions:
Intramuscular injection in cattle, sheep, and swine may result in a local tissue reaction which persists beyond the withdrawal period of 14 days (cattle), 9 days (sheep), or 7 days (swine). This may result in trim loss of edible tissue at slaughter.
Allergic or anaphylactic reactions, sometimes fatal, have been known to occur in animals hypersensitive to penicillin and procaine. Such reactions can occur unpredictably with varying intensity. Animals administered penicillin G procaine should be kept under close observation for at least one half hour. Should allergic or anaphylactic reactions occur, discontinue use of the product and call a veterinarian. If respiratory distress is severe, immediate injection of epinephrine or antihistamine following manufacturer's recommendations may be necessary.
As with all antibiotic preparations, use of this drug may result in overgrowth of nonsusceptible organisms, including fungi. A lack of response by the treated animal, or the development of new signs or symptoms suggest that an overgrowth of nonsusceptible organisms has occurred. In such instances, consult your veterinarian.
It is advisable to avoid giving penicillin in conjunction with bacteriostatic drugs such as tetracyclines.
To report a suspected adverse reaction call 1-866-591-5777.
Storage Conditions:
Bactracillin G should be stored between 2 to 8°C (36 to 46°F).
Restricted Drug - California. Use Only as Directed.
Manufactured for:
Aspen Veterinary Resources,® Ltd.,
Liberty, MO 64068, USA
www.aspenveterinaryresources.com
Made in the UK.
Bactracillin G is a registered trademark of Aspen Veterinary Resources®, LTD. All rights reserved.
006628I01 A0106.NB 11/22
aspen
VETERINARY RESOURCES,® LTD.
Principal Display Panel - 500 Ml Vial Label
NDC 46066-022-05
aspen
VETERINARY RESOURCES®, LTD.
BACTRACILLIN G®
(penicillin G procaine
injectable suspension)
300,000 UNITS PER mL
ANTIBIOTIC
FOR INTRAMUSCULAR INJECTION ONLY
CAUTION: Federal law restricts this drug to use
by or on the order of a licensed veterinarian.
Approved by FDA under NADA # 065-010
NET CONTENTS:
500 mL (16.9 fl oz)
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.