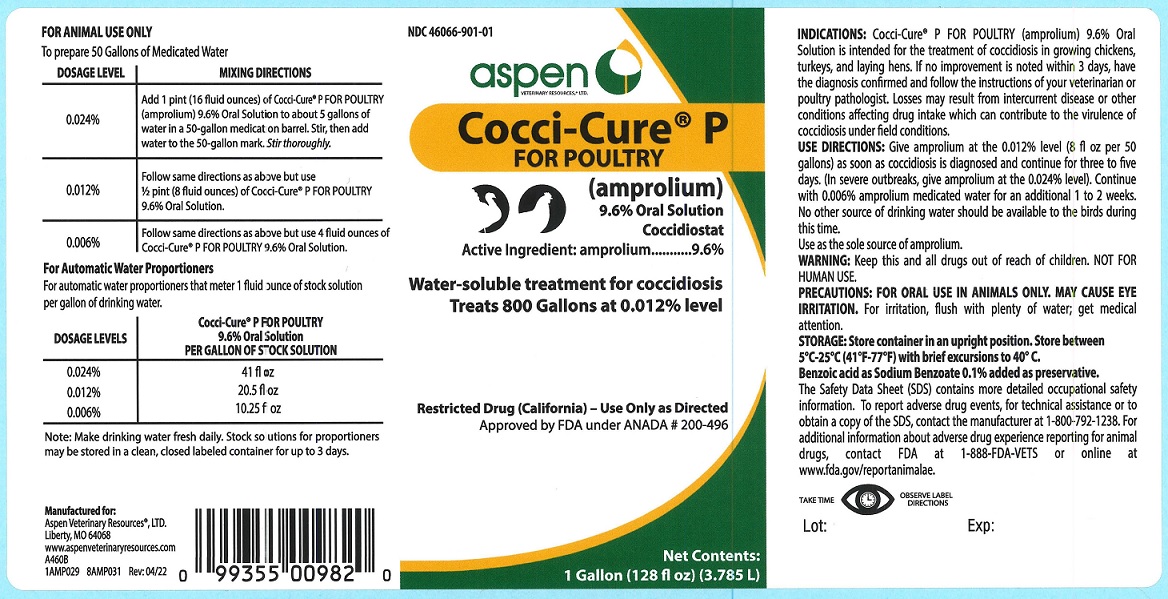
Animal NDC 46066-901-01 Cocci Cure P For Poultry
Amprolium
Animal Product Information
Cocci Cure P For Poultry Images
Cocci Cure P For Poultry Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
Description
Cocci-Cure® P FOR POULTRY
(amprolium)
9.6% Oral Solution
Coccidiostat
Active ingredient: amprolium...........9.6%
Water-soluble treatment for coccidiosis
Treats 800 Gallons at 0.012% level
Restricted Drug (California) - Use Only as Directed
Approved by FDA under ANADA # 200-496
Net Contents: 1 Gallon (128 fl oz)(3.785 L)
Indications & Usage
INDICATIONS: Cocci-Cure® P FOR POULTRY (amprolium) 9.6% Oral Solution is intended for the treatment of coccidiosis in growing chickens, turkeys, and laying hens. If no improvement is noted within 3 days, have the diagnosis confirmed and follow the instructions of your veterinarian or poultry pathologist. Losses may result from intercurrent disease or other conditions affecting drug intake which can contribute to the virulence of coccidiosis under field conditions.
USE DIRECTIONS: Give amprolium at the 0.012% level (8 fl oz per 50 gallons) as soon as coccidiosis is diagnosed and continue for three to five days. (In severe outbreaks, give amprolium at the 0.024% level). Continue with 0.006% amprolium medicated water for an additional 1 to 2 weeks. No other source of drinking water should be available to the birds during this time.
Use as the sole source of amprolium.
Warnings And Precautions
WARNING: Keep this and all drugs out of reach of children. NOT FOR HUMAN USE.
PRECAUTIONS: FOR ORAL USE IN ANIMALS ONLY. MAY CAUSE EYE IRRITATION. For irritation, flush with plenty of water; get medical attention.
Dosage & Administration
FOR ANIMAL USE ONLY
To prepare 50 Gallons of Medicated Water
For Automatic Water Proportioners
For automatic water proportioners that meter 1 fluid ounce of stock solution per gallon of drinking water.
Note: Make drinking water fresh daily. Stock solutions for proportioners may be stored in a clean, closed labeled container for up to 3 days.
Storage And Handling
STORAGE: Store container in an upright position. Store between 5°C-25°C (41°F-77°F) with brief excursions to 40°C.
Benzoic acid as Sodium Benzoate 0.1% added as preservative.
The Safety Data Sheet (SDS) contains more detailed occupational safety information. To report adverse drug events, for technical assistance or to obtain a copy of the SDS, contact the manufacturer at 1-800-792-1238. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.