Animal NDC 51072-041-16 Barrier Ii Wound Care With Pain Relief
Povidone-iodine, Lidocaine, And Alcohol
Animal Product Information
Barrier Ii Wound Care With Pain Relief Images
Barrier Ii Wound Care With Pain Relief Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
Indications
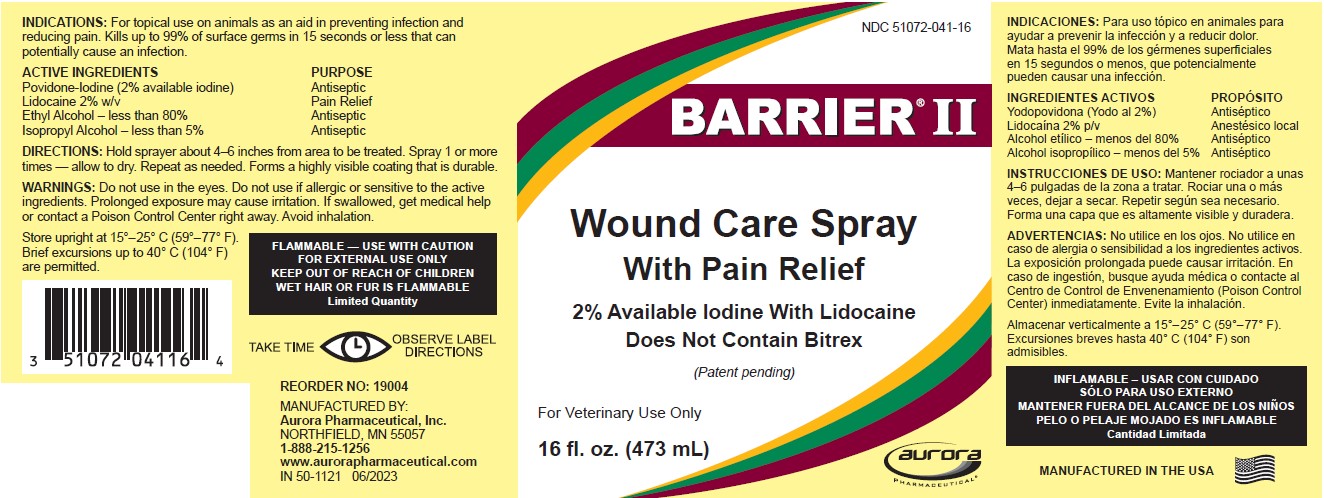
For topical use on animals as an aid in preventing infection and reducing pain. Kills up to 99% of surface germs in 15 seconds or less that can potentially cause an infection.
Description
| ACTIVE INGREDIENTS | PURPOSE |
|---|---|
| Povidone-Iodine (2% available iodine) | Antiseptic |
| Lidocaine 2% w/v | Pain Relief |
| Ethyl Alcohol – less than 80% | Antiseptic |
| Isopropyl Alcohol – less than 5% | Antiseptic |
Directions
Hold sprayer about 4-6 inches from area to be treated. Spray 1 or more times - allow to dry. Repeat as needed. Forms a highly visible coating that is durable.
Warnings
Do not use in the eyes. Do not use if allergic or sensitive to the active ingredients. Prolonged exposure may cause irritation. If swallowed, get medical help or contact a Poison Control Center right away. Avoid inhalation.
Storage And Handling
Store upright at 15°–25° C (59°–77° F). Brief excursions up to 40° C (104° F) are permitted.
Boxed Warning
FLAMMABLE — USE WITH CAUTION
FOR EXTERNAL USE ONLY
KEEP OUT OF REACH OF CHILDREN
WET HAIR OR FUR IS FLAMMABLE
Limited Quantity
Other
REORDER
NO: 19004
MANUFACTURED BY:
Aurora Pharmaceutical, Inc.
NORTHFIELD, MN 55057
1-888-215-1256
www.aurorapharmaceutical.com
IN 50-1121 06/2023
MANUFACTURED IN THE USA
Principal Display Panel - 473 Ml Bottle Label
NDC 51072-041-16
BARRIER II®
Wound Care Spray
With Pain Relief
2% Available Iodine with Lidocaine
Does Not Contain Bitrex
(Patent pending)
For Veterinary Use Only
16 fl. oz. (473 ml)
AURORA PHARMACEUTICAL ®
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.