Animal NDC 51311-141-20 Clomicalm
Clomipramine Hydrochloride
Animal Product Information
Clomicalm Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
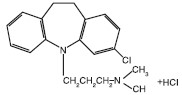
CLOMICALM® (clomipramine hydrochloride) tablets belong to the dibenzazepine class of tricyclic antidepressants. Clomipramine hydrochloride is 3-chloro-5[3-(dimethyl-amino)propyl]-10,11dihydro-5H dibenz[b,f]azepine monohydrochloride. CLOMICALM tablets are oblong, light brown in color and contain clomipramine hydrochloride formulated together with meat components. The molecular weight of clomipramine hydrochloride is 351.3.
Clinical Pharmacology
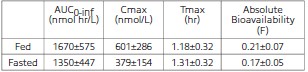
Clomipramine hydrochloride reduces the clinical signs of separation anxiety by affecting serotonergic and noradrenergic neuronal transmission in the central nervous system. While clomipramine hydrochloride can cause lethargy in dogs (see Adverse Reactions) its mode of action is not as a sedative. Clomipramine hydrochloride’s capacity to inhibit re-uptake of serotonin in the central nervous system is believed to be the primary mechanism of action. Clomipramine hydrochloride is rapidly absorbed when administered orally. A single-dose crossover study involving 12 dogs evaluated clomipramine hydrochloride bioavailability after IV (2 mg/kg) and oral (4 mg/kg) administration in either a fed or fasted state. The administration of clomipramine hydrochloride in the presence of food resulted in an increase in the rate and extent of drug absorption as shown in the following table (mean ±SD):
The absolute bioavailability is approximately 25% greater in fed dogs. The apparent terminal plasma half-life ranges from approximately 2 to 9 hours in fed and 3 to 21 hours in fasted dogs. The difference and variability in apparent half-life estimates may be attributable to prolonged drug absorption in the fasted state. The relatively large volume of distribution (3.8±0.8 L/kg) suggests that the drug is widely distributed throughout the body. Clomipramine is primarily metabolized in the liver.
Indications and Usage: CLOMICALM tablets are to be used as part of a comprehensive behavioral management program to treat separation anxiety in dogs greater than 6 months of age. Inappropriate barking or destructive behavior, as well as inappropriate elimination (urination or defecation) may be alleviated by the use of CLOMICALM tablets in conjunction with behavior modification.
Separation anxiety is a complex behavior disorder displayed when the owner (or other attachment figure) leaves the dog. The signs of separation anxiety evaluated in controlled trials were vocalization, destructive behavior, excessive salivation, and inappropriate elimination. In the absence of the owner or attachment figure, dogs with separation anxiety may exhibit one or more of these clinical signs. Although the owner (attachment figure) may inadvertently misinterpret this behavior, which only happens in their absence, as spiteful, it is thought to be the result of anxiety experienced by the dog. Punishment is not considered appropriate for a dog with separation anxiety.
Proper recognition of clinical signs, including a complete patient history and assessment of the patient’s household environment, is essential to accurately diagnose and treat separation anxiety.
The use of CLOMICALM tablets should not replace appropriate behavioral and environmental management but should be used to facilitate a comprehensive behavior management program.
Contraindications
CLOMICALM tablets are contraindicated in dogs with known hypersensitivity to clomipramine or other tricyclic antidepressants.
CLOMICALM tablets should not be used in male breeding dogs. Testicular hypoplasia was seen in dogs treated for 1 year at 12.5 times the maximum daily dose.
CLOMICALM tablets should not be given in combination, or within 14 days before or after treatment with a monoamine oxidase inhibitor [e.g., selegiline hydrochloride (L-deprenyl), amitraz].
CLOMICALM tablets are contraindicated for use in dogs with a history of seizures or concomitantly with drugs which lower the seizure threshold.
Human Warnings
Not for use in humans. Keep out of reach of children. In case of accidental ingestion seek medical attention immediately. In children, accidental ingestion should be regarded as serious. There is no specific antidote for clomipramine. Overdose in humans causes anticholinergic effects including effects on the central nervous (e.g., convulsions) and cardiovascular (e.g., arrhythmia, tachycardia) systems. People with known hypersensitivity to clomipramine should administer the product with caution.
Precautions
General: CLOMICALM tablets are not recommended for other behavior problems, such as aggression (see Adverse Reactions). Studies to establish the safety and efficacy of CLOMICALM tablets in dogs less than 6 months of age have not been conducted.
Diagnosis: It is critical to conduct a comprehensive physical examination, including appropriate laboratory tests, and to obtain a thorough history and assessment of the patient’s household environment, to rule-out causes of inappropriate behavior unrelated to separation anxiety before prescribing CLOMICALM tablets. Periodic reassessment of hematological and serum biochemical data during the administration of this medication is advised. Veterinarians should be familiar with the risks and benefits of the treatment of behavioral disorders in dogs before initiating therapy. Inappropriate use of CLOMICALM tablets, i.e., in the absence of a diagnosis or without concurrent behavioral modification, may expose the animal to unnecessary adverse effects and may not provide any lasting benefit of therapy.
Drug Interactions: Recommendations on the interaction between clomipramine and other medications are extrapolated from data generated in humans. Plasma levels of tricyclic antidepressants have been reported to be decreased by the concomitant administration of hepatic enzyme inducers (e.g., barbiturates, phenytoin); therefore plasma concentrations of clomipramine may be decreased by the concomitant administration of phenobarbital. Plasma levels of closely related tricyclic antidepressants have been reported to be increased by the concomitant administration of hepatic enzyme inhibitors (e.g., cimetidine, fluoxetine). Tricyclic antidepressants themselves may exhibit hepatic enzyme inhibition and possibly increase plasma levels of barbiturates (phenobarbital). Caution is advised in using clomipramine with anticholinergic or sympathomimetic drugs or with other CNS-active drugs, including general anesthetics and neuroleptics. Prior to elective surgery with general anesthetics, clomipramine should be discontinued for as long as clinically feasible.
Use in Concomitant Illness: Use with caution in dogs with cardiovascular disease. At 20 mg/kg/day (5X the maximum recommended dose), bradycardia and arrhythmias (atrioventricular node block and ventricular extrasystole) were observed in dogs. Because of its anticholinergic properties, clomipramine should be used with caution in patients with increased intraocular pressure, a history of narrow angle glaucoma, urinary retention or reduced gastrointestinal motility. Because clomipramine is principally metabolized in the liver, caution is advised in using this medication in the presence of preexisting liver disease.
Reproductive Safety: Safety studies to determine the effects of CLOMICALM tablets in pregnant or lactating female dogs have not been conducted. CLOMICALM tablets should not be used in breeding males (See Contraindications).
Efficacy
Dose Establishment: A 12 week, placebo-controlled, multi-site clinical trial was conducted in the US and Europe to establish an effective dose of CLOMICALM (clomipramine hydrochloride) tablets in dogs. Treatment with CLOMICALM tablets, at 2 - 4 mg/kg/day divided twice daily, in conjunction with behavioral modification (desensitization and counterconditioning) was more effective than behavior modification alone inreducing the signs of separation anxiety in dogs.
Dose Confirmation: In another placebo-controlled, multi-site clinical trial, CLOMICALM tablets at 2 - 4 mg/kg/day given either once daily or divided twice daily showed significant improvement in resolving signs of separation anxiety when tested against behavioral modification alone (desensitization and counterconditioning). In this 8 week study, the rate of improvement of the dogs receiving CLOMICALM tablets with behavioral modification was significantly faster than the rate of improvement of the dogs receiving behavioral modification alone. After one week on trial, 47% of the dogs receiving CLOMICALM tablets once or twice (divided dose) daily in conjunction with behavioral modification showed clinical improvement compared to improvement in 29% of the dogs receiving behavioral modification alone.
Safety
CLOMICALM tablets were demonstrated to be well-tolerated in dogs at the recommended label dose of 2-4 mg/kg/day. In a six month target animal safety study, beagle dogs were dosed daily at 4 (1X), 12 (3X), and 20 (5X) mg/kg/day. Emesis was seen in all groups including the dogs receiving placebo, but occurred more frequently in dogs receiving 12 and 20 mg/kg. Decreased activity was also seen in dogs receiving the 12 and 20 mg/kg. There were no apparent treatment-related alterations in the following: body weights, physical examination findings, electrocardiograph examinations, hematology or biochemistry parameters, ophthalmoscopic examinations, macroscopic or microscopic organ examinations and organ weights. Average food and water consumption over the 26 week period was similar for control and treated groups. In a one year study, pure bred dogs were dosed daily at 12.5 (3X), 50 (12.5X), and 100 (25X) mg/kg/day. Emesis and mydriasis were observed within 15 minutes to one hour after dosing in dogs receiving 12.5, 50, and 100 mg/kg/day and lethargy was observed within
1 hour of dosing in dogs receiving 50 and 100 mg/kg. Testicular hypoplasia was seen in dogs receiving 50 mg/kg. At 100 mg/kg/day (25X) convulsions and eventual death occurred in five out of the eight dogs.
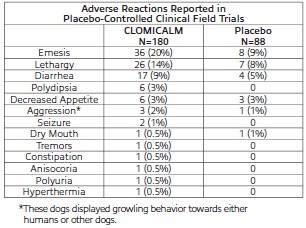
Adverse Reactions
Frequency and category of adverse reactions observed in dogs dosed with CLOMICALM tablets or placebo were observed in multisite clinical studies as follows.
Post-Approval Experience: Although not all adverse reactions are reported, the following adverse reactions are based on voluntary post-approval adverse drug experience reporting: lethargy/depression, anorexia, elevation in liver enzymes, vomiting and diarrhea. Hepatobiliary disease has occurred, especially in the presence of pre-existing conditions or with concurrent administration of drugs metabolized via the hepatic system. Additionally, in an overdose situation, the following signs have been reported: ataxia, convulsion(s), anticholinergic effects (e.g., mydriasis, bradycardia, tachycardia, and arrhythmia) and vocalization.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Virbac AH, Inc. at 1-800-338-3659 or us.virbac.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at
1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
Dosage And Administration
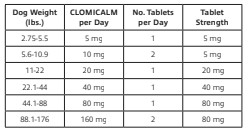
The recommended daily dose of CLOMICALM tablets is 2 to 4 mg/kg/day (0.9 -1.8 mg/lb/day) (see dosing table below). It can be administered as a single daily dose or divided twice daily based on patient response and/or tolerance of the side effects. It may be prudent to initiate treatment in divided doses to minimize side effects by permitting tolerance to side effects to develop or allowing the patient time to adapt if tolerance does not develop. To reduce the incidence of vomiting that may be experienced by some dogs, CLOMICALM tablets may be given with a small amount of food.
The specific methods of behavioral modification used in clinical trials involved desensitization and counterconditioning techniques. Since the manifestation of separation anxiety can vary according to the individual dog, it is advised that a specific behavior modification plan be developed based on a professional assessment of each individual case.
Once the desired clinical effect is achieved and the owners have successfully instituted the appropriate behavioral modification, the dose of CLOMICALM tablets may be reduced to maintain the desired effect or discontinued. Withdrawal side effects were not reported in studies with CLOMICALM tablets in dogs. However, in clinical practice, it is recommended to taper the individual patient dose while continuing to monitor the dog’s behavior and clinical status through the dose reduction or withdrawal period. Continued behavioral modification is recommended to prevent recurrence of the clinical signs.
The effectiveness and clinical safety of CLOMICALM tablets for long-term use (i.e., for more than 12 weeks) has not been evaluated.
Professional judgment should be used in monitoring the patient’s clinical status, response to therapy and tolerance to side effects to determine the need to continue treatment with CLOMICALM tablets and to continue to rule-out physiological disorders which may complicate the diagnosis and treatment of separation anxiety.
Storage Conditions
Store in a dry place at controlled room temperature, between 59° and 77°F (15-25°C). Store unused tablets in the original closed container.
How Supplied
CLOMICALM tablets are available in 5, 20, 40 and 80 mg tablet strengths in color-coded packaging for oral administration to dogs.
Keep this and all drugs out of reach of children.
Other
Manufactured by: Virbac AH, Inc.
P.O. Box 162059, Fort Worth, TX 76161, USA
Approved by FDA under NADA # 141-120.
09/22 -02
302304
© 2022 Virbac Corporation All rights reserved. CLOMICALM is a registered trademark of Virbac Group of Companies.
Package Label.Principal Display Panel
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.