Animal NDC 54771-1547-1 Mga 500
Melengestrol Acetate
Animal Product Information
| Field Name | Field Value |
|---|---|
| Animal NDC Code | 54771-1547-1 |
| Proprietary Name | Mga 500 What is the Proprietary Name? The proprietary name also known as the trade name is the name of the product chosen by the medication labeler for marketing purposes. |
| Non-Proprietary Name | Melengestrol Acetate What is the Non-Proprietary Name? The non-proprietary name is sometimes called the generic name. The generic name usually includes the active ingredient(s) of the product. |
| Labeler Name | Zoetis Inc. |
| Product Type | Otc Animal Drug |
| Usage Information |
|
| Active Ingredient(s) |
|
| Marketing Category | NADA - NEW ANIMAL DRUG APPLICATION What is the Marketing Category? Product types are broken down into several potential Marketing Categories, such as NDA/ANDA/BLA, OTC Monograph, or Unapproved Drug. One and only one Marketing Category may be chosen for a product, not all marketing categories are available to all product types. Currently, only final marketed product categories are included. The complete list of codes and translations can be found at www.fda.gov/edrls under Structured Product Labeling Resources. |
| FDA Application Number | NADA039402 What is the FDA Application Number? This corresponds to the NDA, ANDA, or BLA number reported by the labeler for products which have the corresponding Marketing Category designated. If the designated Marketing Category is OTC Monograph Final or OTC Monograph Not Final, then the Application number will be the CFR citation corresponding to the appropriate Monograph (e.g. “part 341”). For unapproved drugs, this field will be null. |
Mga 500 Images
Mga 500 Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
Directions For Use
Heifers Fed in Confinement for Slaughter:
MGA 500 (melengestrol acetate Type A liquid medicated article) should be thoroughly mixed in liquid Type C medicated feed which must be fed at 0.5 to 2.0 pounds per head daily to provide 0.25 to 0.5 mg of melengestrol acetate per head per day. Average daily intakes approximating the middle of this range provide the most optimal and economical improvements in rate of gain and feed utilization. Constant daily intakes of 0.35 to 0.50 mg per head per day give a high degree of estrus suppression. Levels of 0.25 to 0.35 mg provide a lower but still effective degree of estrus suppression.
Heifers Intended for Breeding:
MGA 500 should be thoroughly mixed in the supplement to provide 0.5 mg of melengestrol acetate per head per day.
WARNINGS: Not for human use. Restricted Drug (California)—Use Only As Directed
PRECAUTIONS: Not effective in steers and spayed heifers.
Heifers Fed in Confinement for Slaughter:
Withdrawal periods of three to five days or more should be avoided to prevent the possibility that the heifers may come into estrus (heat) at loading time.
Heifers Intended for Breeding:
Do not exceed 24 days of feeding of melengestrol acetate to heifers intended for breeding. A reduced conception rate can be expected if heifers are bred at estruses observed within 1 to 12 days after withdrawal of melengestrol acetate, whereas heifers bred at subsequent observed estruses are expected to have normal conception rates.
Mixing Directions
Liquid Type B and C medicated feeds containing melengestrol acetate must have a pH of 4.0 to 8.0 and their labels must bear appropriate mixing directions. Mixing directions for liquid Type B or C feeds stored in recirculation tank systems are: “Recirculate immediately prior to use for no less than 10 minutes, moving not less than 1 percent of the tank contents per minute from the bottom of the tank to the top. Recirculate daily, as directed in this paragraph even when the Type B (or C) feed is not used.” Mixing directions for liquid Type B and C feeds stored in mechanical, air or other agitation-type tank systems are: “Agitate immediately prior to use for not less than 10 minutes, creating a turbulence at the bottom of the tank that is visible at the top. Agitate daily, as directed in this paragraph, even when the Type B (or C) feed is not used.”
Intermediate medicated feeds should not be made from MGA 500 except as a part of a continuous mixing operation to make a complete liquid Type B or Type C medicated feed.
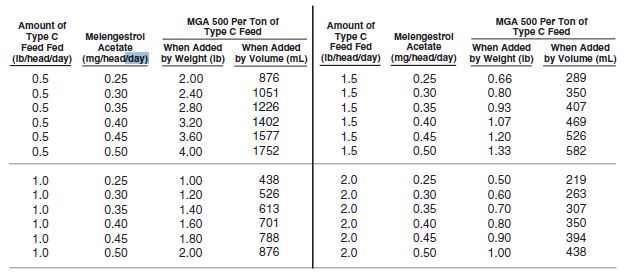
Thoroughly mix 0.5 to 4 pounds of MGA 500 per ton of a non-medicated feed to prepare a Type C medicated feed containing 0.25 to 2.0 grams of melengestrol acetate per ton. The following Table may be used as a guide in determining the amount of MGA 500 to be added to prepare a ton of Type C medicated feed.
Type B medicated feed containing 4 to 10 grams melengestrol acetate per ton may be manufactured by thoroughly mixing 8 to 20 lbs of MGA 500 (melengestrol acetate Type A liquid medicated article) with 1992 to 1980 lbs of non-medicated feed. Labeling for such Type B feed shall contain directions for manufacturing Type C medicated feeds containing 0.25 to 2.0 grams melengestrol acetate per ton (0.125 to 1.0 mg/lb). The Type C medicated feed, containing melengestrol acetate, must be top dressed on grain or roughage or mixed with a complete ration at the rate of 0.5 to 2.0 pounds per head per day. Good manufacturing practice regulations must be adhered to in manufacturing feeds containing MGA 500.
Principal Display Panel - Bag Label
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.