Animal NDC 54771-1906-1 Bonqat
Pregabalin
Animal Product Information
Bonqat Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
Description
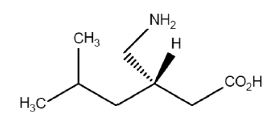
Each mL of BONQAT contains 50 mg pregabalin. The chemical name of pregabalin is (3S)-3-(aminomethyl)-5-methylhexanoic acid. It is a white or almost white powder having a molecular weight of 159.23g /mol. The molecular formula is C8H17NO2 and the structural formula is:
Indications
BONQAT is indicated for alleviation of acute anxiety and fear associated with transportation and veterinary visits in cats.
Dosage And Administration
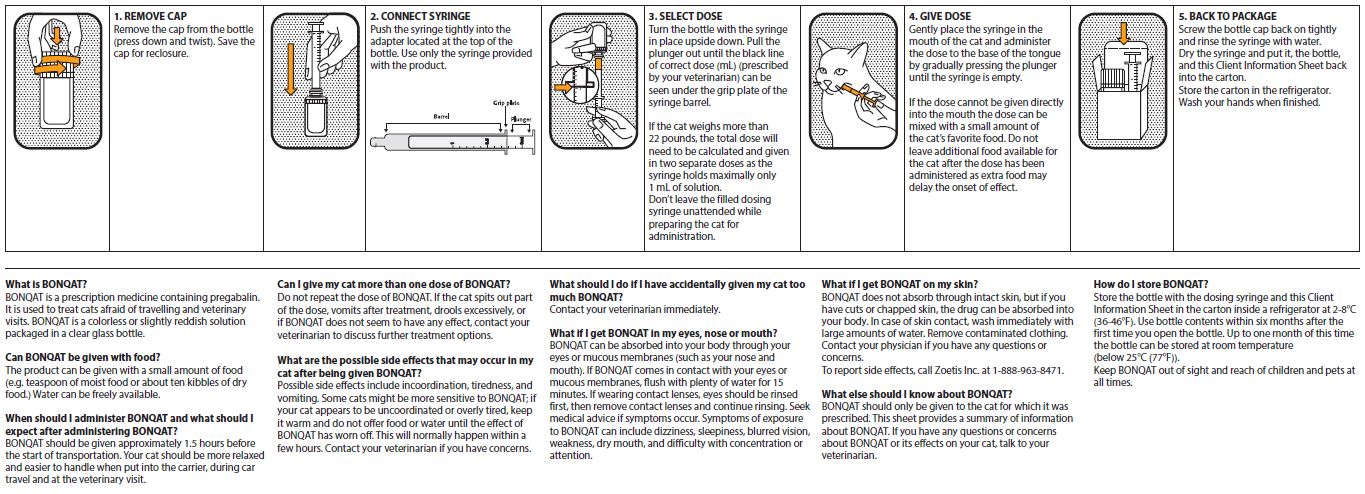
Client Information Sheet is on the reverse of this package insert.
Always provide client information sheet with prescription.
BONQAT is administered orally as a single dose of 5 mg/kg (0.1 mL/kg) approximately 1.5 hours before the start of the transportation or veterinary visit and can be given on two consecutive days.
If the cat weighs more than 22 pounds, the total dose will need to be calculated and given in two separate doses as the syringe holds a maximum of 1 mL of solution.
A small amount of food can be given with BONQAT.
Contraindications
Do not use in case of hypersensitivity to pregabalin or to any of the excipients (sodium benzoate, ethyl maltol, hydrochloric acid, sodium hydroxide).
Warnings
Human Safety Warnings:
Not for human use.
Appropriate precautions should be taken while handling BONQAT. Avoid skin contact, eye contact, or contact with mucous membranes.
Symptoms of exposure to pregabalin include dizziness, sleepiness, blurred vision, weakness, dry mouth, and difficulty with concentration or attention.
In case of accidental eye or mucosal exposure, flush with water for 15 minutes. If wearing contact lenses, eyes should be rinsed first, then remove contact lenses and continue rinsing. Seek medical advice if symptoms occur.
In case of skin contact, wash with soap and water immediately. Remove contaminated clothing. Seek medical advice if symptoms occur.
In case of accidental ingestion, seek medical advice if symptoms occur. Do not drive as sleepiness may occur. In case of ingestion by a child, seek medical attention immediately. Show the package insert or the label to the physician.
Drug Abuse, Addiction, and Diversion
Controlled Substance:
BONQAT contains pregabalin, a Schedule V controlled substance.
Abuse: Abuse is defined as the intentional, non-therapeutic use of a drug, even once, to achieve a desired psychological or physiological effect. Pregabalin is not known to be active at receptor sites associated with drugs of abuse. However, pregabalin is associated with drug liking and is known to be misused and abused in the community, particularly in combination with opioids. Consider the potential risks of misuse or abuse before prescribing this product. Signs of pregabalin misuse or abuse include drug seeking behavior.
Pregabalin should be handled appropriately to minimize the risk of diversion, including restriction of access, the use of accounting procedures, and proper disposal methods, as appropriate to the clinical setting and as required by law.
Note to physician: BONQAT contains pregabalin.
The safety data sheet (SDS) contains more detailed occupational safety information. To report adverse reactions in users or to obtain a copy of the SDS for BONQAT contact Zoetis Inc. at 1-888-963-8471.
Animal Safety Warnings: Some cats may experience hypothermia, depression, drowsiness, muscle tremors, and/or ataxia. These cats should be kept warm and not offered food or water until BONQAT’s effects have worn off (usually within 6 hours). (See TARGET ANIMAL SAFETY)
Keep BONQAT in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
Precautions
Use with caution in cats with concurrent cardiac disease or hypertension because BONQAT may cause bradycardia and reflex hypertension. The safety of BONQAT has not been evaluated in cats with concurrent cardiac disease or hypertension.
The safe use of BONQAT in cats younger than 7 months of age has not been evaluated.
The safe use of BONQAT used in conjunction with opioids and other sedatives has not been evaluated.
Use with caution in cats with pre-existing renal disease (See Clinical Pharmacology).
The safe use of BONQAT in cats with severe systemic disorders has not been evaluated. Use with caution in cats with severe systemic disorder.
The safe use of BONQAT in breeding, pregnant, and lactating cats has not been evaluated.
Adverse Reactions
In a well-controlled European field study, which included a total of 238 cats (108 treated with BONQAT at the label dose of 5 mg/kg, 29 treated with BONQAT at a dose of 2.5 mg/kg, and 101 administered placebo control), 5 months to 15 years of age and weighing 1.8 to 10.3 kg, the following adverse reactions were reported:
Table 1. Adverse reactions
| Adverse reaction | Pregabalin | Pregabalin 2.5 mg/kg N=29 | Placebo N=101 |
| Ataxia | 5 (4.6%) | 1 (3.4%) | 0 |
| Lethargy | 3 (2.8%) | 2 (6.9%) | 0 |
| Emesis | 2 (1.9%) | 0 | 0 |
| Proprioception abnormality | 1 (0.9%) | 1 (3.4%) | 0 |
| Muscle tremor | 1 (0.9%) | 0 | 0 |
| Anorexia | 1 (0.9%) | 0 | 0 |
| Weight loss | 1 (0.9%) | 0 | 0 |
| Mydriasis | 0 | 1 (3.4%) | 0 |
Contact Information
To report suspected adverse events, for technical assistance or to obtain a copy of the SDS, contact Zoetis Inc. at 1-888-963-8471.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
Information For Cat Owners
Possible side effects on BONQAT include incoordination, tiredness, and vomiting. Some cats might be more sensitive to BONQAT; if the cat appears to be uncoordinated or overly tired, it should be kept warm and not offered food or water until the effect of BONQAT has worn off. This will normally happen within a few hours. If there are further concerns related to side effects after dosing the veterinarian should be contacted.
BONQAT must not be re-dosed if the cat spits part of the dose, vomits after treatment, or in case of hypersalivation, or if BONQAT does not seem to have any effect.
Always provide the Client Information Sheet with prescription.
Keep BONQAT in a secure location out of reach of children, dogs, cats, and other animals to prevent accidental ingestion or overdose.
Clinical Pharmacology
Absorption
BONQAT is rapidly absorbed after oral administration in cats. Following oral administration of 5 mg/kg to fasted cats the maximum observed concentration (Cmax) in plasma was 10.1 ± 0.8 (SD) μg/mL and occurred at 0.5-1.0 hours post-dose. The area under plasma concentration time curve from time zero to the last quantifiable time point (AUClast) was 199±27 μg*h/mL. The mean absolute oral bioavailability was 94.3%.
Distribution
BONQAT has a relatively large volume of distribution. After intravenous (IV) bolus administration of 2.5 mg/kg, the volume of distribution at the steady state (Vss) was 0.4±0.02 L/kg in cats. BONQAT is not known to bind to plasma proteins in other species, but this has not been studied in cats.
Metabolism and excretion
BONQAT is slowly eliminated from the body of cats. After IV bolus administration of 2.5 mg/kg, total plasma clearance was 0.03±0.008 L/h/kg. The mean half-life of elimination from circulation was 12.3±3.1 hours after IV administration of 2.5 mg/kg and 14.7±2.7 hours after oral administration of 5 mg/kg.
Elimination of the parent compound as well as the methylation metabolite from circulation occurs almost exclusively by renal excretion in other species. This has not been studied in cats.
Mechanism of Action
Pregabalin is a ligand of alpha2-delta subunit of voltage-gated calcium channels. It reduces the presynaptic calcium influx in neurons and thereby the release of various neurotransmitters, including glutamate, norepinephrine, serotonin, dopamine, substance P and calcitonin gene-related peptide.
Effectiveness
The effectiveness of BONQAT was demonstrated in a well-controlled multi-center field study conducted in Europe. A total of 238 cats with a history of being anxious and/or fearful when transported by a car and during veterinary visits were allocated to 1 of 3 treatment groups: 108 cats received BONQAT orally at a dose of 5 mg/kg, 29 cats received BONQAT at a dose of 2.5 mg/kg and 101 cats received placebo (vehicle oral solution containing no active ingredient). The study included assessments during transportation and veterinary examination at a screening visit and a treatment visit. To keep both study visits as similar as possible, all cats were administered tap water prior to the screening visit. Prior to the treatment visit, each cat was administered its randomized treatment. The treatment visit was conducted 5-10 days after the screening visit in a similar manner as the screening visit. Doses were administered approximately 1.5 hours before the cat was put into the carrier and starting of the transportation to the visits. The cats were between 5 months and 15 years of age and weighed 1.8 to 10.3 kg.
Treatment success was defined with 2 primary effectiveness endpoints: the owner’s assessment of the treatment effect based on the cat’s anxiety and/or fear during transportation in a car and the veterinarian’s assessment of the treatment effect based on the cat’s anxiety and/or fear during physical examination at the clinic. These endpoints were based on the following scale (1- Excellent, 2- Good, 3- Fair, 4- Poor, 5- Very Poor).
The treatment effect was statistically significant for both primary effectiveness variables (p < 0.0010) in the group administered BONQAT at the dose 5 mg/kg compared to the placebo group. In the 5 mg/kg group, cat owners assessed the response as excellent or good during the transportation in 54.3% of cases; the corresponding proportion in the placebo group was 27.1%. In the 5 mg/kg group, the veterinarians assessed the response as excellent or good during the clinical examination in 52% of cases; the corresponding proportion in the placebo group was 30%. The 2.5 mg/kg group was not statistically different than placebo for both primary variables.
The owner’s assessment of ability to place the cat into the carrier was improved from screening to treatment visit in the group administered BONQAT at the dose of 5 mg/kg compared to the placebo group. The owner-assessed signs of anxiety and/or fear decreased from screening to treatment visit in the 5 mg/kg group compared to the placebo group . Based on the owners’ observations, vocalization, panting/intense breathing, activity, resistance, and freezing were the signs with the greatest improvement between screening and treatment visits in the 5 mg/kg group. The majority of the cat owners assessed administration of BONQAT as either very easy or easy.
Target Animal Safety
In a margin of safety study, 32 healthy, 7-month-old Domestic shorthair cats (4/sex/group) were administered a negative control or BONQAT for six consecutive days by once daily oral administration at dose levels of 5, 15, and 25 mg/kg/day.
At 5 mg/kg/day, observed signs of sedation included: abnormal gait, slight to moderate uncoordinated behavior, decreased activity, slightly limited usage of hind limbs, lying on side, hypothermia and/or drowsy appearance (i.e. depression, drowsiness, and/or ataxia). Clinical signs of sedation were resolved at the four-hour clinical observation. One male and one female cat had hypothermia observed two to four hours post-dose respectively, the lowest body temperature value was 99°F. Cats had a decrease in heart rate with maximum effect at six hours, but the heart rates stayed within normal range. All adverse observations were resolved by six hours after dosing on the first day of treatment.
At 15 and 25 mg/kg/day, signs of sedation were observed in all cats and included ataxia, lethargy, slightly to moderately limited usage of hind limbs, slight to severe uncoordinated behavior, partially to completely closed eyes, lying on side, dilated pupils, hypothermia, and/or drowsy appearance (i.e. depression, drowsiness, and/or ataxia). On Day 1, all cats had a decreased body temperature at one or more timepoints, the lowest values were 97.8°F when dosed at 15 mg/kg/day and 98.2°F when dosed at 25 mg/kg/day. One cat dosed at 25 mg/kg/day had a loss of consciousness, abnormal gait, eyes closed, decreased activity, lying on side, sedation, salivation, vomiting, hypothermia, and uncoordinated behavior. This cat recovered by the four-hour observation. Directly after dosing, slight to severe salivation was observed in multiple cats on one or more days. Cats had decreased heart rate with maximum effect at two to six hours, some cats had bradycardia (120-130 bpm). The majority of cats maintained a normal blood pressure, but a few cats with bradycardia had a reflexive hypertension. Most adverse observations resolved by eight hours after treatment administration.
In a second margin of safety study, 32 healthy, 1 to 3 years old Domestic shorthair cats (4/sex/group) were administered a negative control or BONQAT for 3 consecutive days by once daily oral administration at dose levels of 5, 15 and 25 mg/kg/day. Directly after dosing, slight to severe hypersalivation was noted in all dose groups.
At 5 mg/kg/day, signs of sedation were observed in 6 of 8 cats, and included: abnormal gait, hypothermia, decreased respiratory rate and/or lethargy. These signs were observed between 1 and 6 hours after dosing on the first day of treatment. On Day 2, at six hours post-dose, one cat had muscle tremors that resolved without treatment by the 8-hour observation. Three cats had bradycardia (120-128 bpm) with maximum effect from two to six hours post-dose, but the heart rate remained within the normal range for the other five cats.
At 15 and 25 mg/kg/day, signs of sedation observed in all cats included: ataxia, hypothermia, lethargy, uncoordinated behavior, decreased respiratory rate, and/or they were cold to the touch. The signs of sedation were observed for 12 hours after dosing. One cat in the 15 mg/kg/day dose group had muscle tremors at four hours post-dosing as well as ataxia, lethargy, hypothermia, and a decrease in heart rate. Cats had a decrease in heart rate with maximum effect at two to six hours, a few cats had bradycardia (106-122 bpm). The majority of cats maintained a normal blood pressure, but a few cats with bradycardia had a reflex hypertension. One cat in the 15 mg/kg/day dose group had bradycardia with reflex hypertension at two hours post-dose followed by a reflex tachycardia at six- and eight-hours post-dose.
Storage Information
Store the bottle in a refrigerator at 2-8°C (36-46°F). Use bottle contents within six months after the first opening of the bottle. Up to one month of this time the bottle can be stored at or below 25°C (77°F).
How Supplied
BONQAT is packed in a clear 5 mL glass bottle containing 2 mL of BONQAT (pregabalin oral solution) at 50 mg/mL. The bottle has a child resistant closure and an oral dosing syringe adapter. The bottle is further packed into a carton with a package insert and an oral dosing syringe (1 mL).
Information Sheet
Bonqat® CV
(pregabalin oral solution)
CLIENT INFORMATION SHEET FOR OWNER/HANDLER USE AND SAFETY
This sheet summarizes the basic information about BONQAT and does not replace the instructions from your veterinarian.
Talk to your veterinarian if you have questions regarding any part of this information or if you want to know more about BONQAT.
INSTRUCTIONS FOR USE
Orion Pharma
Zoetis
Principal Display Panel
160463
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.