Animal NDC 54771-3139-9 Albadry Plus
Novobiocin Sodium And Penicillin G Procaine
Animal Product Information
Albadry Plus Images
Albadry Plus Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
| Each 10 mL PLASTET® Disposable Syringe contains: | |
|---|---|
| Novobiocin sodium equiv. to novobiocin | 400 mg |
| Penicillin G procaine | 200,000 IU |
| Chlorobutanol anhydrous (chloral derivative—used as a preservative) in a special bland vehicle | 50 mg |
Manufactured by a non-sterilizing process.
Indications For Use
ALBADRY PLUS Suspension is indicated for the treatment, in dry cows only, of subclinical mastitis caused by susceptible strains of Staphylococcus aureus and Streptococcus agalactiae.
Warnings
1. Do not use less than 30 days prior to calving.
2. Milk from treated cows must not be used for food during the first 72 hours after calving.
3. Treated animals must not be slaughtered for food for 30 days following udder infusion.
Precaution
Administration of this product in any manner other than shown under DOSAGE may result in drug residues.
Dosage
Infuse one tube per quarter at start of dry period (but not less than 30 days prior to calving).
Shake Well Before Using
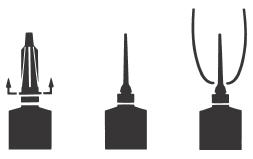
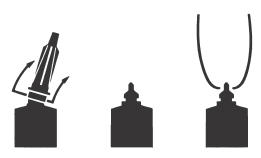
Directions For Using The Flexi-Tube® System
The FLEXI-TUBE is designed to provide the choice of either insertion of the full cannula, as has traditionally been practiced, or insertion of no more than ⅛ inch of the cannula, as recommended by the National Mastitis Council.
- Full Insertion: Remove the blue end cap by pulling straight up as shown. Gently insert the full cannula into the teat canal; carefully infuse the product.
- Partial Insertion: Remove both the blue end cap and the red cannula by pushing sideways as shown. Gently insert the exposed blue tip into the teat canal; carefully infuse the product.
Administration
At the time of drying off, but not less than 30 days prior to calving, milk the udder dry. Wash the teats and udder thoroughly with warm water containing a suitable dairy antiseptic. Dry the teats and udder thoroughly. Infuse each quarter using the following procedure. Using the alcohol pads provided, scrub each teat end clean using a separate pad for each teat. Warm ALBADRY PLUS Suspension to body temperature and shake thoroughly. Choose the desired insertion length (full or partial) and insert tip into teat canal. Instill entire contents into the quarter. Massage the udder after treatment to distribute the ALBADRY PLUS Suspension throughout the quarters. Using a suitable teat dip, dip all teats following treatment.
Storage Conditions
Store at controlled room temperature 20° to 25° C (68° to 77° F).
How Supplied
ALBADRY PLUS Suspension is available in unbroken packages of 12–10 mL PLASTET Disposable Syringes with 12 individually wrapped 70% isopropyl alcohol pads and unbroken packages of 144–10 mL PLASTET Disposable Syringes with 144 individually wrapped 70% isopropyl alcohol pads.
Principal Display Panel - 10 Ml Syringe Label
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.