Animal NDC 54771-5193-1 Doxirobe Gel
Doxycycline Hyclate
Animal Product Information
Doxirobe Gel Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
DOXIROBE Gel is provided in a 2-syringe system requiring mixing prior to use. Syringe A contains the polymer delivery system (N-methyl-2-pyrrolidone and poly (DL-lactide)) and Syringe B contains the active ingredient (doxycycline). Once mixed, the product is a flowable solution of doxycycline hyclate equivalent to 8.5% doxycycline activity. The formulation is applied subgingivally to the periodontal pocket(s) of affected teeth, and doxycycline is slowly released from the polymer providing a local antimicrobial effect. The product is non-irritating and biodegradable.
Clinical Pharmacology
Doxycycline is a semi-synthetic tetracycline derivative. Consistent with the tetracycline class of antibiotics, it has a wide range of antimicrobial activity against microorganisms. Upon contact with the aqueous environment (gingival crevicular fluid) the polymer will coagulate, resulting in the formation of a solid, pliable delivery system within the treated periodontal pocket(s). Doxycycline is released into the gingival crevicular fluid for a local effect on the microorganisms, particularly gram-negative anaerobic bacteria, involved in periodontal disease.
In a clinical trial, the highest plasma doxycycline concentration was observed at 6 hours after treatment administration (2-4 affected teeth in each of 6 dogs). Doxycycline was not detected in plasma samples taken at 24 hours after treatment administration or any time point thereafter. All detectable concentrations of doxycycline were well below levels associated with systemic activity or toxicity.
Indications And Usage
DOXIROBE Gel is indicated for the treatment and control of periodontal disease in dogs.
Periodontal pocket probing depths ≥ 4 mm are evidence of disease that may be responsive to treatment with DOXIROBE Gel. In clinical trials, use of the product resulted in attachment level gains, periodontal pocket depth reductions and improved gingival health. Noticeable improvements in these parameters should be evident within 2-4 weeks following treatment. The response in individual animals is dependent on the severity of the condition and rigor of adjunctive therapy. This product is not intended for use in oronasal fistulas, periapical abscesses, or severely compromised teeth.
Precautions
Do not use in dogs less than 1 year of age as the use of tetracyclines during tooth development has been associated with permanent discoloration of the teeth. Do not use in pregnant bitches. The use of the product in breeding dogs has not been evaluated.
Clients should be advised to suspend brushing treated teeth for approximately 2 weeks following treatment.
Dosage And Administration
Teeth should be cleaned and scaled prior to application of the product.
If required, root planing and debridement of affected sites should be performed. The product is applied with the animal under sedation or anesthesia. Use as many units as required to fill the periodontal pockets of affected teeth.
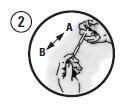
Each pouch contains 2 syringes and a blunt cannula. Syringe A contains the polymer delivery system and Syringe B contains doxycycline. Lock the syringes together.
Beginning with Syringe A, use the plungers of Syringes A and B to exchange the material between the syringes approximately 100 times to achieve a consistent mixture.
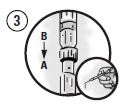
Fully deliver the mixture into Syringe A, separate the syringes, and lock the supplied blunt cannula onto Syringe A. The cannula may be bent to the desired angle.
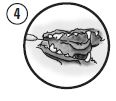
Gently place the cannula 1-2 mm below the gingival margin of an affected tooth. Express a small amount of the mixture into each periodontal pocket 4 mm or deeper. Ensure that the pockets are filled approximately to the gingival margin.
The formulation will begin to solidify immediately upon application; however, lavage with a few drops of water or saline will facilitate the process. Allow approximately 30-60 seconds for the polymer to harden before beginning to press it into the pocket
The exposed surface of the product may be pressed into the pocket with the edge of a wax spatula or the back of a curette. Pressure may be applied to the gingival margin to avoid dislodging the polymer inadvertently. As the product is biodegradable, removal at a subsequent visit is not required.
Treatment and control of periodontal disease requires a comprehensive program of routine scaling and cleaning, home care and dental hygiene (e.g., brushing, rinses or the use of chewing devices) in addition to application of this product. Severe cases may require surgical intervention.
Reconstituted product not used on the day of mixing should be stored in the resealable foil pouch at room temperature (15-25°C or 59-77°F) and used within 3 days of reconstitution. Ten additional exchanges between syringes should be performed if reconstituted product has been stored.
Storage Conditions
Store at controlled room temperature 15° to 30°C (59° to 86°F).
How Supplied
Each unit of DOXIROBE Gel contains Syringe A (polymer delivery system) and Syringe B (doxycycline), which when mixed result in approximately 0.5 ml of doxycycline solution. Available as a 3-unit carton (containing 1 additional blunt cannula), NDC 0009-5193-01.
Principal Display Panel Carton
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.