Animal NDC 54771-8431-1 Albon
Sulfadimethoxine
Animal Product Information
Albon Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Albon is a low-dosage, rapidly absorbed, long-acting sulfonamide, effective for the treatment of a wide range of bacterial infections commonly encountered in dogs and cats.
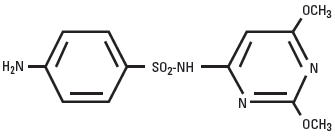
Sulfadimethoxine is a white, almost tasteless and odorless compound. Chemically, it is N1-(2,6-dimethoxy-4-pyrimidinyl) sulfanilamide. The structural formula is:
Action
Sulfadimethoxine has been demonstrated clinically or in the laboratory to be effective against a variety of organisms, such as streptococci, klebsiella, proteus, shigella, staphylococci, escherichia, and salmonella.1,2 These organisms have been demonstrated in respiratory, genitourinary, enteric, and soft tissue infections of dogs and cats.
The systemic sulfonamides which include sulfadimethoxine are bacteriostatic agents. Sulfonamides competitively inhibit bacterial synthesis of folic acid (pteroylglutamic acid) from para-aminobenzoic acid. Mammalian cells are capable of utilizing folic acid in the presence of sulfonamides.
The tissue distribution of sulfadimethoxine, as with all sulfonamides, is a function of plasma levels, degree of plasma protein binding, and subsequent passive distribution in the tissues of the lipid-soluble un-ionized form. The relative amounts are determined by both its pKa and by the pH of each tissue. Therefore, levels tend to be higher in less acid tissue and body fluids or those diseased tissues having high concentrations of leucocytes.2
In the dog, sulfadimethoxine is not acetylated as in most other animals, and it is excreted predominantly as the unchanged drug.3 Sulfadimethoxine has a relatively high solubility at the pH normally occurring in the kidney, precluding the possibility of precipitation and crystalluria. Slow renal excretion results from a high degree of tubular reabsorption,4 and plasma protein binding is very high, providing a blood reservoir of the drug. Thus, sulfadimethoxine maintains higher blood levels than most other long-acting sulfonamides. Single, comparatively low doses of Albon give rapid and sustained therapeutic blood levels.1
To assure successful sulfonamide therapy (1) the drug must be given early in the course of the disease, and it must produce a high sulfonamide level in the body rapidly after administration, (2) therapeutically effective sulfonamide levels must be maintained in the body throughout the treatment period, (3) treatment should continue for a short period of time after the clinical signs have disappeared, and (4) the causative organisms must be sensitive to this class of drugs.
Indications And Usage
Albon is indicated for the treatment of respiratory, genitourinary tract, enteric, and soft tissue infections in dogs and cats:
| tonsillitis pharyngitis bronchitis pneumonia cystitis nephritis metritis pyometra | pustular dermatitis anal gland infections abscesses wound infections bacterial enteritis canine salmonellosis bacterial enteritis associated with coccidiosis in dogs |
when caused by streptococci, staphylococci, escherichia, salmonella, klebsiella, proteus or shigella organisms sensitive to sulfadimethoxine.
Limitations: Sulfadimethoxine is not effective in viral or rickettsial infections, and as with any antibacterial agent, occasional failures in therapy may occur due to resistant microorganisms. The usual precautions in sulfonamide therapy should be observed.
Warning
Not for human use.
Precaution
During treatment period, make certain that animals maintain adequate water intake.
If animals show no improvement within 2 or 3 days, reevaluate your diagnosis.
Dosage And Administration
Initial Dose: 25 mg/lb (55 mg/kg) of animal body weight.
Subsequent Daily Doses: 12.5 mg/lb (27.5 mg/kg) of animal body weight.
For ease of administration in animals of varying weights, 3 tablet sizes are provided. The following table indicates how dosage may be adjusted depending on tablet size and body weight. Subsequent doses should be given at 24-hour intervals.
| Tablet Size | Approximate Animal Weight | Initial Dose 25 mg/lb (55 mg/kg) | Subsequent Daily Doses 12.5 mg/lb (27.5 mg/kg) |
|---|---|---|---|
| 125 mg | 5 lb (2.2 kg) | 1 tablet | 1/2 tablet |
| 250 mg | 10 lb (4.5 kg) | 1 tablet | 1/2 tablet |
| 500 mg | 20 lb (9.1 kg) | 1 tablet | 1/2 tablet |
Treatment may be initiated with Albon Injection 40% to obtain effective blood levels almost immediately or to facilitate treatment of the fractious animal.
Length of treatment depends on the clinical response. In most cases treatment for 3–5 days is adequate. Treatment should be continued until the animal is asymptomatic for 48 hours.
Toxicity And Safety
Data regarding acute and chronic toxicities of sulfadimethoxine indicate the drug is very safe. The LD50 in mice is greater than 2 g/kg of body weight when administered intraperitoneally and greater than 16 g/kg when administered orally. In dogs receiving massive single oral doses of 3.2 g/kg of body weight, diarrhea was the only adverse effect observed. Dogs given 160 mg/kg of body weight orally daily for 13 weeks showed no signs of toxicity.
Storage
Store at controlled room temperature 15°–30°C (59°–86°F).
How Supplied
Albon Tablets are available in the following strengths for dogs and cats: 125 mg, 250 mg, or 500 mg sulfadimethoxine per tablet.
References
1. Data on file, Pfizer Animal Health.
2. Stowe CM: The sulfonamides. In Jones LM (ed), Veterinary Pharmacology and Therapeutics, Ames, Iowa, Iowa State University Press, chapter 33, 1965.
3. Bridges JW, Kirby MR, Walker SR, et al: Species differences in the metabolism of sulfadimethoxine. Biochem J 109:851, 1968.
4. Baggot JD: Some aspects of drug persistence in domestic animals. Res Vet Sci 11(2):130, 1970.
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.