Animal NDC 69043-016-01 Clindamycin
Animal Product Information
Clindamycin Animal Product Labeling Information
The product labeling information includes all published material associated to a drug. Product labeling documents include information like generic names, active ingredients, ingredient strength dosage, routes of administration, appearance, usage, warnings, inactive ingredients, etc.
Table of Contents
Description
Clindamycin Hydrochloride Capsules, USP contain clindamycin hydrochloride which is the hydrated salt of clindamycin. Clindamycin is a semisynthetic antibiotic produced by a 7(S)-chlorosubstitution of the 7(R)-hydroxyl group of a naturally produced antibiotic produced by Streptomyces lincolnensis var. lincolnensis.
Clindamycin Hydrochloride Capsules, USP (For Use in Dogs Only):
25 mg Capsule, each yellow and colorless capsule contains clindamycin hydrochloride equivalent to 25 mg of clindamycin.
75 mg Capsule, each green and colorless capsule contains clindamycin hydrochloride equivalent to 75 mg of clindamycin.
150 mg Capsule, each blue and colorless capsule contains clindamycin hydrochloride equivalent to 150 mg of clindamycin.
300 mg Capsule, each turquoise and colorless capsule contains clindamycin hydrochloride equivalent to 300 mg of clindamycin.
Clinical Pharmacology
Absorption
Clindamycin hydrochloride is rapidly absorbed from the canine gastrointestinal tract.
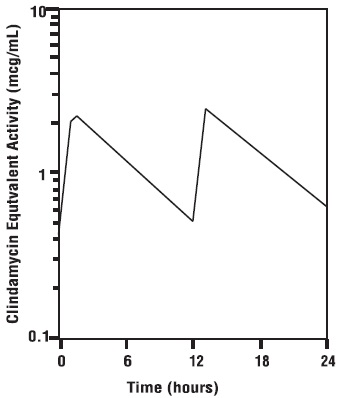
Dog Serum Levels
Serum levels at or above 0.5 µg/mL can be maintained by oral dosing at a rate of 2.5 mg/lb of clindamycin hydrochloride every 12 hours. This same study revealed that average peak serum concentrations of clindamycin occur 1 hour and 15 minutes after oral dosing. The elimination half -life for clindamycin in dog serum was approximately 5 hours. There was no bioactivity accumulation after a regimen of multiple oral doses in healthy dogs.
Clindamycin Serum Concentrations
2.5 mg/lb (5.5 mg/kg) After B.I.D. Oral
Dose of Clindamycin Hydrochloride Capsules to Dogs
Metabolism and Excretion
Extensive studies of the metabolism and excretion of clindamycin hydrochloride administered orally in animals and humans have shown that unchanged drug and bioactive and bioinactive metabolites are excreted in urine and feces. Almost all of the bioactivity detected in serum after clindamycin hydrochloride product administration is due to the parent molecule (clindamycin). Urine bioactivity, however, reflects a mixture of clindamycin and active metabolites, especially N-demethyl clindamycin and clindamycin sulfoxide.
Site and Mode of Action
Clindamycin is an inhibitor of protein synthesis in the bacterial cell. The site of binding appears to be in the 50S sub-unit of the ribosome. Binding occurs to the soluble RNA fraction of certain ribosomes, thereby inhibiting the binding of amino acids to those ribosomes. Clindamycin differs from cell wall inhibitors in that it causes irreversible modification of the protein-synthesizing subcellular elements at the ribosomal level.
Microbiology
Clindamycin is a lincosaminide antimicrobial agent with activity against a wide variety of aerobic and anaerobic bacterial pathogens. Clindamycin is a bacteriostatic compound that inhibits bacterial protein synthesis by binding to the 50S ribosomal sub-unit. The minimum inhibitory concentrations (MICs) of Gram-positive and obligate anaerobic pathogens isolated from dogs in the United States are presented in Table 1. Bacteria were isolated in 1998-1999. All MICs were performed in accordance with the Clinical and Laboratory Standards Institute (CLSI).
| Organism | Number of Isolates | MIC50 | MIC85 | MIC90 | Range |
| Soft Tissue/Wound2 | |||||
| Staphylococcus aureus | 17 | 0.5 | 0.5 | ≥ 4.0 | 0.25- ≥ 4.0 |
| Staphylococcus intermedius | 28 | 0.25 | 0.5 | ≥ 4.0 | 0.125- ≥ 4.0 |
| Staphylococcus spp | 18 | 0.5 | 0.5 | ≥ 4.0 | 0.25- ≥ 4.0 |
| Beta-hemolytic streptococci | 46 | 0.5 | 0.5 | ≥ 4.0 | 0.25- ≥ 4.0 |
| Streptococcus spp | 11 | 0.5 | ≥ 4.0 | ≥ 4.0 | 0.25-≥ 4.0 |
| Osteomyelitis/Bone3 | |||||
| Staphylococcus aureus | 20 | 0.5 | 0.5 | 0.5 | 0.54 |
| Staphylococcus intermedius | 15 | 0.5 | ≥ 4.0 | ≥ 4.0 | 0.25-≥ 4.0 |
| Staphylococcus spp | 18 | 0.5 | ≥ 4.0 | ≥ 4.0 | 0.25-≥ 4.0 |
| Beta-hemolytic streptococci | 21 | 0.5 | 2.0 | 2.0 | 0.25-≥ 4.0 |
| Streptococcus spp | 21 | ≥ 4.0 | ≥ 4.0 | ≥ 4.0 | 0.25-≥ 4.0 |
| Dermal/Skin5 | |||||
| Staphylococcus aureus | 25 | 0.5 | ≥ 4.0 | ≥ 4.0 | 0.25-≥ 4.0 |
| Staphylococcus intermedius | 48 | 0.5 | ≥ 4.0 | ≥ 4.0 | 0.125- ≥ 4.0 |
| Staphylococcus spp | 32 | 0.5 | ≥ 4.0 | ≥ 4.0 | 0.25-≥ 4.0 |
| Beta-hemolytic streptococci | 17 | 0.5 | 0.5 | 0.5 | 0.25-0.5 |
1 The correlation between the in vitro susceptibility data and clinical response has not been determined.
2 Soft Tissue/Wound: includes samples labeled wound, abscess, aspirate, exudates, draining tract, lesion, and mass.
3 Osteomyelitis/Bone: includes samples labeled bone, fracture, joint, tendon
4 No range, all isolates yielded the same value
5 Dermal/Skin: includes labeled skin, skin swab, biopsy, incision, lip
Indications
Clindamycin Hydrochloride Capsules, USP (for use in dogs only) are indicated for the treatment of infections caused by susceptible strains of the designated microorganisms in the specific conditions listed below:
Dogs: Skin infections (wounds and abscesses) due to coagulase positive staphylococci (Staphylococcus aureus or Staphylococcus intermedius).Deep wounds and abscesses due to Bacteroides fragilis, Prevotella melaninogenicus, Fusobacterium necrophorum and Clostridium perfringens.
Dental infections due to Staphylococcus aureus, Bacteroides fragilis, Prevotella melaninogenicus, Fusobacterium necrophorum and Clostridium perfringens.Osteomyelitis due to Staphylococcus aureus, Bacteroides fragilis, Prevotella melaninogenicus, Fusobacterium necrophorum and Clostridium perfringens.
Contraindications
Clindamycin Hydrochloride Capsules, USP are contraindicated in animals with a history of hypersensitivity to preparations containing clindamycin or lincomycin.
Because of potential adverse gastrointestinal effects, do not administer to rabbits, hamsters, guinea pigs, horses, chinchillas or ruminating animals.
Warnings
Keep out of reach of children. Not for human use.
Precautions
During prolonged therapy of one month or greater, periodic liver and kidney function tests and blood counts should be performed.
The use of Clindamycin Hydrochloride Capsules, USP occasionally results in overgrowth of non-susceptible organisms such as clostridia and yeasts. Therefore, the administration of Clindamycin Hydrochloride Capsules should be avoided in those species sensitive to the gastrointestinal effects of clindamycin (see CONTRAINDICATIONS). Should superinfections occur, appropriate measures should be taken as indicated by the clinical situation.
Patients with very severe renal disease and/or very severe hepatic disease accompanied by severe metabolic aberrations should be dosed with caution, and serum clindamycin levels monitored during high-dose therapy.
Clindamycin hydrochloride has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. Therefore, Clindamycin Hydrochloride Capsules, USP should be used with caution in animals receiving such agents.
Safety in gestating bitches and queens or breeding male dogs has not been established.
Adverse Reactions
Side effects occasionally observed in either clinical trials or during clinical use were vomiting and diarrhea.
To report a suspected adverse reaction call 1-844-227-6687 (1-844-2-CRONUS)
Dosage And Administration
Dogs: Infected Wounds, Abscesses, and Dental Infections
Oral: 2.5-15.0 mg/lb body weight every 12 hours.
Duration: Treatment with Clindamycin Hydrochloride Capsules, USP may be continued up to a maximum of 28 days if clinical judgment indicates. Treatment of acute infections should not be continued for more than three or four days if no response to therapy is seen.
Dosage Schedule
Capsules
Clindamycin Hydrochloride Capsules, USP 25 mg, administer 1-6 capsules every 12 hours for each 10 pounds of body weight.
Clindamycin Hydrochloride Capsules, USP 75 mg, administer 1 -6 capsules every 12 hours for each 30 pounds of body weight.
Clindamycin Hydrochloride Capsules, USP 150 mg, administer 1-6 capsules every 12 hours for each 60 pounds of body weight.
Clindamycin Hydrochloride Capsules, USP 300 mg, administer 1-6 capsules every 12 hours for each 120 pounds of body weight.
Dogs: Osteomyelitis
Oral: 5.0-15.0 mg/lb body weight every 12 hours.
Duration: Treatment with Clindamycin Hydrochloride Capsules is recommended for a minimum of 28 days. Treatment should not be continued for longer than 28 days if no response to therapy is seen.
Dosage Schedule:
Capsules
Clindamycin Hydrochloride Capsules, USP 25 mg, administer 2-6 capsules every 12 hours for each 10 pounds of body weight.
Clindamycin Hydrochloride Capsules, USP 75 mg, administer 2 -6 capsules every 12 hours for each 30 pounds of body weight.
Clindamycin Hydrochloride Capsules, USP 150 mg, administer 2-6 capsules every 12 hours for each 60 pounds of body weight.
Clindamycin Hydrochloride Capsules, USP 300 mg, administer 2-6 capsules every 12 hours for each 120 pounds of body weight
Animal Safety Summary
Rat and Dog Data: One year oral toxicity studies in rats and dogs at doses of 30, 100 and 300 mg/kg/day (13.6, 45.5 and 136.4 mg/lb/day) have shown clindamycin hydrochloride capsules to be well tolerated. Differences did not occur in the parameters evaluated to assess toxicity when comparing groups of treated animals with contemporary controls. Rats administered clindamycin hydrochloride at 600 mg/kg/day (272.7 mg/lb/day) for six months tolerated the drug well; however, dogs orally dosed at 600 mg/kg/day (272.7 mg/lb/day) vomited, had anorexia, and subsequently lost weight. At necropsy these dogs had erosive gastritis and focal areas of necrosis of the mucosa of the gallbladder.
Safety in gestating bitches or breeding males has not been established.
Storage
Store at controlled room temperature 68° to 77°F (20° to 25°C).
How Supplied
Clindamycin Hydrochloride Capsules are available as:
25 mg - bottles of 200
75 mg - bottles of 200
150 mg - bottles of 100 and 500
300 mg - bottles of 100
To report suspected adverse drug events, for technical assistance or to obtain a copy of the safety data sheet (SDS), contact Cronus Pharma LLC at 1-844-227-6687 or 1-844-2-CRONUS.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at https://www.fda.gov/animal-veterinary/safety-health.
Approved by FDA under ANADA # 200-298
Manufactured for:
Cronus Pharma LLC,
East Brunswick, NJ 08816.
Contact No: 1-844-227-6687
(1-844-2-CRONUS)
Made in India
Distributed by:
Cronus Pharma LLC
2 Tower Center Boulevard, Suite 1101A,
East Brunswick, NJ 08816
Contact No: 1-844-227-6687 (1-844-2-CRONUS)
e-Fax No: 732-647-1272
Email: [email protected]
Issued: 02/2023
Package Label.Principal Display Panel
NDC 69043-013-20
Clindamycin Hydrochloride Capsules, USP
25 mg
Equivalent to 25 mg clindamycin
Approved by FDA under ANADA # 200-298
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
200 Capsules
For Use in Dogs Only
NDC 69043-014-20
Clindamycin Hydrochloride Capsules, USP
75 mg
Equivalent to 75 mg clindamycin
Approved by FDA under ANADA # 200-298
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
200 Capsules
For Use in Dogs Only
NDC 69043-015-01
Clindamycin Hydrochloride Capsules, USP
150 mg
Equivalent to 150 mg clindamycin
Approved by FDA under ANADA # 200-298
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
100 Capsules
For Use in Dogs Only
NDC 69043-015-05
Clindamycin Hydrochloride Capsules, USP
150 mg
Equivalent to 150 mg clindamycin
Approved by FDA under ANADA # 200-298
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
500 Capsules
For Use in Dogs Only
NDC 69043-016-01
Clindamycin Hydrochloride Capsules, USP
300 mg
Equivalent to 300 mg clindamycin
Approved by FDA under ANADA # 200-298
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
100 Capsules
For Use in Dogs Only
* The information on this page is for an ANIMAL PRODUCT, please review the complete disclaimer below.