Pill Identifier Adderall XR - NDC 54092-389
Dextroamphetamine Sulfate, Dextroamphetamine Saccharate, Amphetamine Sulfate And Amphetamine Aspartate
Product Code 54092-389
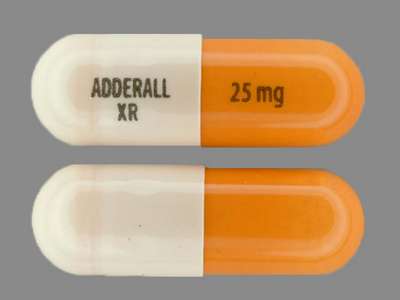
Adderall XR by Takeda Pharmaceuticals America, Inc. is a orange and white capsule capsule extended release about 21 mm in size, imprinted with adderall;xr;25;mg. The product is a human prescription drug with active ingredient(s) dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine sulfate and amphetamine aspartate.
Adderall XR
Active Ingredient(s): Dextroamphetamine Sulfate, Dextroamphetamine Saccharate, Amphetamine Sulfate And Amphetamine Aspartate
Inactive Ingredient(s):
- GELATIN, UNSPECIFIED
- HYPROMELLOSE, UNSPECIFIED
- METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1)
- SUCROSE
- TALC
- TRIETHYL CITRATE
- FERRIC OXIDE RED
- TITANIUM DIOXIDE
- FERRIC OXIDE YELLOW
Takeda Pharmaceuticals America, Inc.
Product Type: Human Prescription Drug
Dosage Form: Capsule, Extended Release
- Orange (C48331)
- White (C48325)
DEA Schedule: CII
Product Packages
| NDC Package Code | Package Description |
|---|---|
| 54092-389-01 | 100 CAPSULE, EXTENDED RELEASE in 1 BOTTLE |
* Please review the disclaimer below.
Pill Identification data last updated on: December, 2018
This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product. If this is an emergency, call 911 or the national Poison Help hotline at 1-800-222-1222. Most images are not part of the product labels and have not been verified by the labeler company. ALWAYS consult your physician or pharmacist with any medication-related questions.