Product Images Ellence
View Photos of Packaging, Labels & Appearance
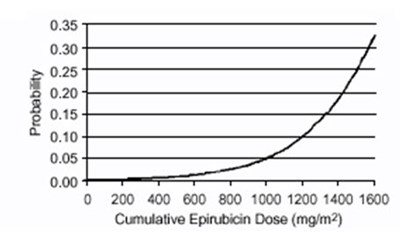
- Figure 1 - ellence 01
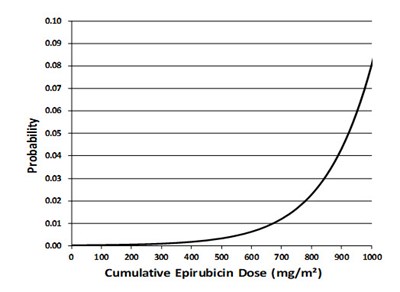
- Figure 2 - ellence 02
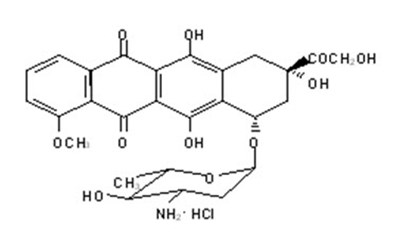
- Chemical Structure - ellence 03
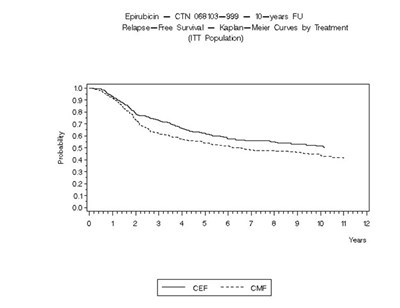
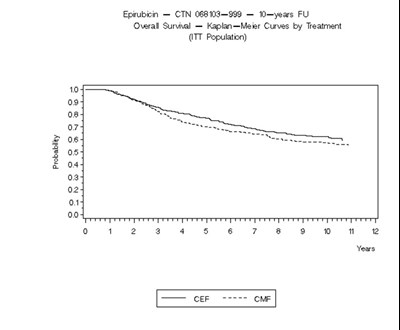
- Figure 3 - ellence 04
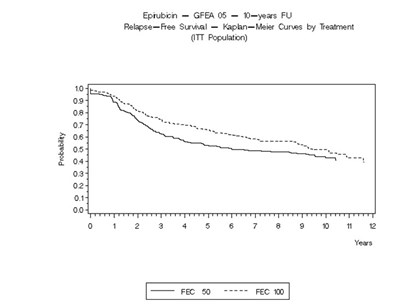
- Figure 4 - ellence 05
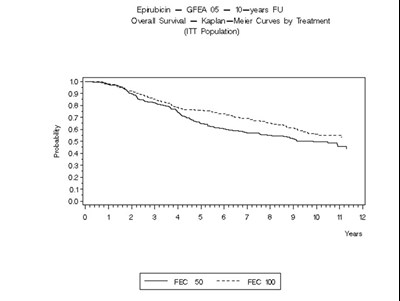
- Figure 5 - ellence 06
- Figure 6 - ellence 07
- Logo - ellence 08
- PRINCIPAL DISPLAY PANEL - 50 mg/25 mL Vial Label - ellence 09
- PRINCIPAL DISPLAY PANEL - 50 mg/25 mL Vial Carton - ellence 10
- PRINCIPAL DISPLAY PANEL - 200 mg/100 mL Vial Label - ellence 11
- PRINCIPAL DISPLAY PANEL - 200 mg/100 mL Vial Carton - ellence 12
Product Label Images
The following 12 images provide visual information about the product associated with Ellence NDC 0009-5093 by Pharmacia & Upjohn Company Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Logo - ellence 08

This is a product label for injectables distributed by Pharmacia & Upjohn Co, a division of Pfizer Inc, located in New York City.*
PRINCIPAL DISPLAY PANEL - 50 mg/25 mL Vial Label - ellence 09

This is a pharmaceutical product called Ellence, which is an injectable containing epirubicin hydrochloride for intravenous use only. It is available in a single-dose vial and should be kept refrigerated between 2-8°C. This product is manufactured in Australia and distributed by Pharmacia & Upjohn Co. Further details on dosage and use can be found in the accompanying prescribing information.*
PRINCIPAL DISPLAY PANEL - 50 mg/25 mL Vial Carton - ellence 10
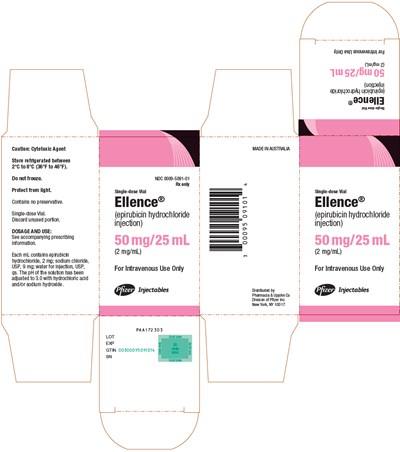
This is a cautionary label for a cytotoxic agent that is required to be refrigerated and protected from light. The vial contains epirubicin hydrochloride, sodium chloride, and water for injection, and has a pH of 3.0. The dosage and usage instructions should be found in the accompanying prescribing information. This text does not provide the prescription information.*
PRINCIPAL DISPLAY PANEL - 200 mg/100 mL Vial Label - ellence 11

This is a description of a medication called Ellence (epirubicin hydrochloride injection) indicated for intravenous use only. The medication is stored refrigerated between 2°C to 8°C and should not be frozen to be used as injectable product. It should be protected from light and discarded after a single use. The text also provides information on dosage, usage, and prescribing information. The product is distributed by Pharmacia & Upjohn Co Division of Pfizer Inc in New York, NY. The lot number of this medication is PAN72290.*
PRINCIPAL DISPLAY PANEL - 200 mg/100 mL Vial Carton - ellence 12
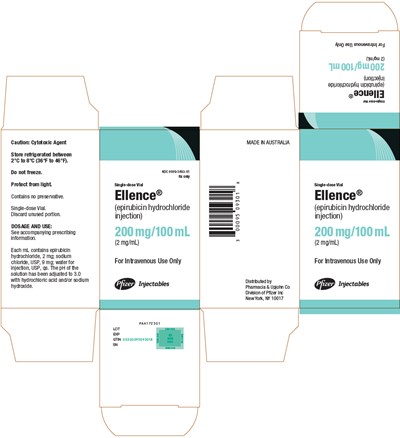
This is a caution regarding a cytotoxic agent that needs to be stored in a refrigerated environment. It is also advised to protect the agent from light and not to freeze. The solution has no preservatives and comes in a single-dose vial, so the unused portion should be discarded. The solution contains epirubicin hydrochloride, sodium chloride, and water for injection. The pH of the solution has been adjusted to 3.0 with hydrochloric acid and/or sodium hydroxide. The accompanying prescribing information should be consulted for dosage and use. It is for intravenous use only. The agent is good for a 100mL dose and has 200 mg of epirubicin hydrochloride per 100mL of solution. It is distributed by Pfizer Inc. and is made in Australia.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.