Product Images Caverject Impulse
View Photos of Packaging, Labels & Appearance
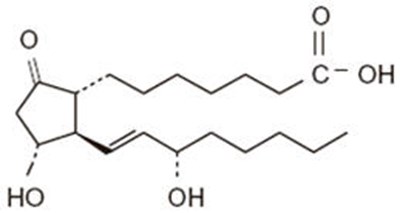
- Chemical Structure - caverject 01
- Logo - caverject 02
- Figure A - caverject 03
- exclaim image - caverject 04
- Figure B - caverject 05
- Figure C - caverject 06
- Figure D - caverject 07
- Figure E - caverject 08
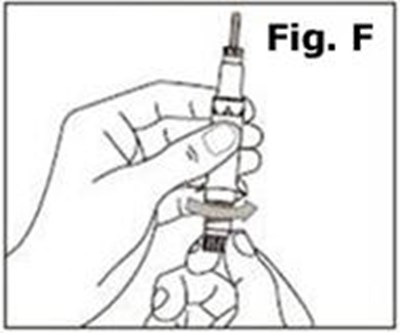
- Figure F - caverject 09
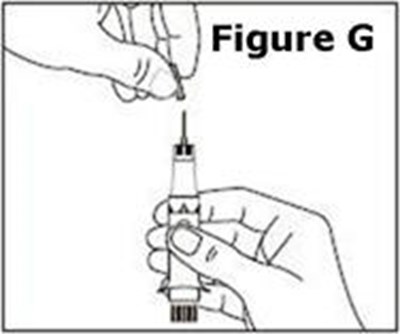
- Figure G - caverject 10
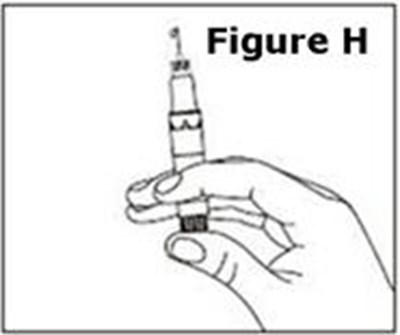
- Figure H - caverject 11
- Figure I - caverject 12
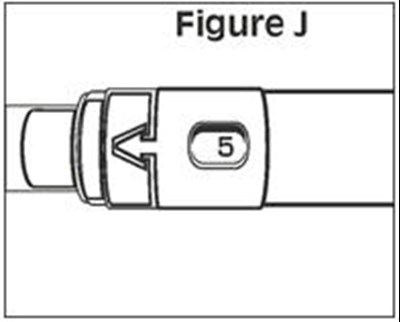
- Figure J - caverject 13
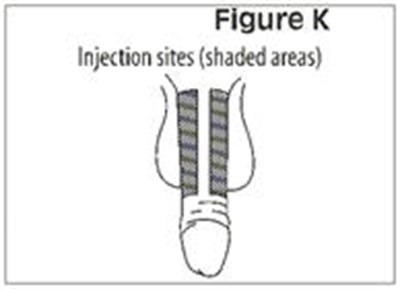
- Figure K - caverject 14
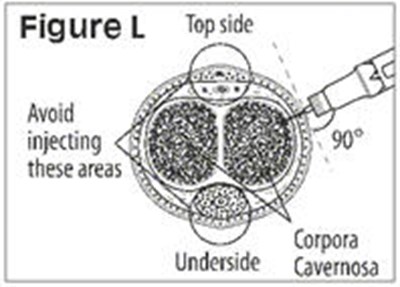
- Figure L - caverject 15
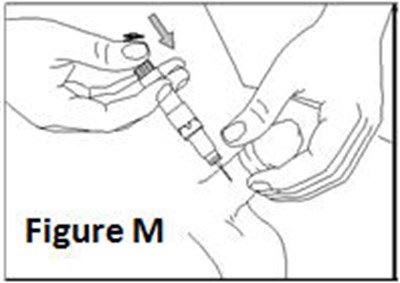
- Figure M - caverject 16
- Principal Display Panel - 10 microgram Syringe Label - caverject 17
- Principal Display Panel - 10 micrograms Syringe Carton - caverject 18
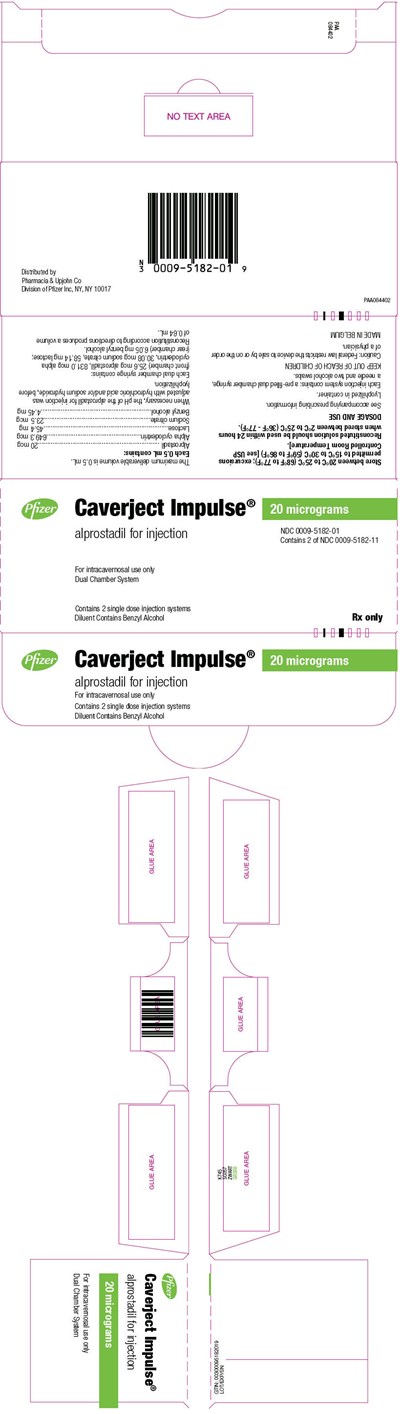
- Principal Display Panel - 20 microgram Syringe Label - caverject 19
- Principal Display Panel - 20 micrograms Syringe Carton - caverject 20
Product Label Images
The following 20 images provide visual information about the product associated with Caverject Impulse NDC 0009-5181 by Pharmacia & Upjohn Company Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure A - caverject 03

This appears to be a diagram or image of a "needle assembly" with various parts labeled, including an "outer protective cap", "inner protective cap", "superfine needle", "clear plastic tip", "dose window", and "plunger rod". There is also a mention of a possible "glass cartridge" inside. However, without more context it's unclear what purpose or application this needle assembly serves.*
Principal Display Panel - 10 microgram Syringe Label - caverject 17

This is a description of an injection system called Gaverject Impulse® made by Pharmacia & Upjohn Co, a division of Pfizer Inc, in Belgium. The system includes a dual chamber and is used for injecting alprostadil at a concentration of 10 micrograms. The label warns to keep it out of reach of children. The numbers at the bottom of the label likely refer to lot and expiration information.*
Principal Display Panel - 10 micrograms Syringe Carton - caverject 18

This is a product description for Alprostadil for injection, which is for intracavernosal use only and contains two single-dose injection systems. The package also includes a diluent containing benzyl alcohol and a dual-chamber system. The product is distributed by Pfizer and has two National Drug Codes (NDC): 0009-5181-01 and 0009-5181-10.*
Principal Display Panel - 20 microgram Syringe Label - caverject 19

This is a product label for Caverject Impulse, a dual chamber system for injecting alprostadil, a medication used to treat erectile dysfunction. The label indicates that the product contains 20 micrograms of alprostadil and warns to keep it out of reach of children. The manufacturer is Pharmacia & Upjohn Co, a division of Pfizer Inc., and the product is made in Belgium. The label also includes a lot/exp number for tracking and quality control purposes.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.