FDA Label for Optiray 320
View Indications, Usage & Precautions
- 1 INDICATIONS AND USAGE
- 1.1 INTRA-ARTERIAL
- 1.2 INTRA-VENOUS
- 2.1 IMPORTANT DOSAGE AND ADMINISTRATION INSTRUCTIONS
- 2.2 RADIO FREQUENCY IDENTIFICATION (RFID)-TAGGED SYRINGE DIRECTIONS FOR USE
- 2.3 INTRA-ARTERIAL PROCEDURES IN ADULTS
- CEREBRAL ARTERIOGRAPHY
- 2.4 INTRAVENOUS PROCEDURES IN ADULTS
- 2.5 PEDIATRIC DOSING
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5.1 RISKS ASSOCIATED WITH INADVERTENT INTRATHECAL ADMINISTRATION
- 5.2 HYPERSENSITIVITY REACTIONS
- 5.3 CONTRAST INDUCED ACUTE KIDNEY INJURY
- 5.4 CARDIOVASCULAR ADVERSE REACTIONS
- 5.5 THROMBOEMBOLIC EVENTS
- 5.6 EXTRAVASATION AND INJECTION SITE REACTIONS
- 5.7 THYROID STORM IN PATIENTS WITH HYPERTHYROIDISM
- 5.8 HYPERTENSIVE CRISIS IN PATIENTS WITH PHEOCHROMOCYTOMA
- 5.9 SICKLE CELL CRISIS IN PATIENTS WITH SICKLE CELL DISEASE
- 5.10 SEVERE CUTANEOUS ADVERSE REACTIONS
- 6 ADVERSE REACTIONS
- 6.1 CLINICAL STUDIES EXPERIENCE
- 6.2 POSTMARKETING EXPERIENCE
- 7 DRUG INTERACTIONS
- 7.1 DRUG-DRUG INTERACTIONS
- 7.2 DRUG/LABORATORY TEST INTERACTIONS
- 8.1 PREGNANCY
- 8.2 LACTATION
- 8.4 PEDIATRIC USE
- 8.5 GERIATRIC USE
- 8.6 RENAL IMPAIRMENT
- 10 OVERDOSAGE
- 11 DESCRIPTION
- 11.1 CHEMICAL CHARACTERISTICS
- 11.2 PHYSICAL CHARACTERISTICS
- 12.1 MECHANISM OF ACTION
- 12.2 PHARMACODYNAMICS
- 12.3 PHARMACOKINETICS
- 13.1 CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
- 13.2 ANIMAL TOXICOLOGY AND/OR PHARMACOLOGY
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 16.1 HOW SUPPLIED
- 16.2 STORAGE
- 16.3 HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - OPTIRAY 350, 125 ML SYRINGE LABEL
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - OPTIRAY 320, 125 ML SYRINGE LABEL
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - OPTIRAY 300, 100 ML SYRINGE LABEL
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - OPTIRAY 240, 125 ML SYRINGE LABEL
Optiray 320 Product Label
The following document was submitted to the FDA by the labeler of this product Liebel-flarsheim Company Llc. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
1 Indications And Usage
Optiray is indicated for:
1.1 Intra-Arterial
In adults
- Optiray 240: cerebral arteriography.
- Optiray 300: cerebral arteriography and peripheral arteriography.
- Optiray 320: cerebral arteriography, peripheral arteriography, visceral and renal arteriography, aortography, coronary arteriography, and left ventriculography.
- Optiray 350: peripheral arteriography coronary arteriography, and left ventriculography.
- Optiray 320 and Optiray 350: angiocardiography.
In pediatric patients
1.2 Intra-Venous
In adults
- Optiray 240: Computed tomography (CT) imaging of the head and body, venography, and intravenous excretory urography.
- Optiray 300: CT imaging of the head and body, venography, and intravenous excretory urography.
- Optiray 320: CT imaging of the head and body, venography, and intravenous excretory urography.
- Optiray 350: CT imaging of the head and body, venography, intravenous excretory urography, and intravenous digital subtraction angiography (IV-DSA).
- In pediatric patients Optiray 320: CT imaging of the head and body, and intravenous excretory urography.
2.1 Important Dosage And Administration Instructions
- Optiray is for intravascular use only [see Boxed Warning, Contraindications (4), Warnings and Precautions (5.1)].
- Use sterile technique for all handling and administration of Optiray.
- Inspect glass and plastic containers prior to use for breakage or other damage and do not use damaged containers.
- Warm Optiray and administer at body or room temperature.
- Inspect Optiray for particulate matter or discoloration before administration. Do not administer if Optiray contains particulate matter or is discolored.
- Do not mix Optiray with other drugs, solutions or total parenteral nutrition mixtures.
- Use the lowest dose necessary to obtain adequate visualization.
- Adjust the volume and concentration of Optiray. Modify the dose accounting for factors such as age, body weight, vessel size, blood flow rate within the vessel, anticipated pathology, degree and extent of opacification required, structure(s) or area to be examined, disease processes affecting the patient, and equipment and technique to be employed.
- Avoid extravasation when injecting Optiray; especially in patients with severe arterial or venous disease [see Warnings and Precautions (5.6)].
- Hydrate patients before and after Optiray administration [see Warnings and Precautions ( 5.3 )].
- Discard unused portion of Optiray from single-dose container after use.
2.2 Radio Frequency Identification (Rfid)-Tagged Syringe Directions For Use
- The RFID-tagged syringe must be used with an Optivantage Injector with RFID technology [see How Supplied/Storage and Handling (16.2)]. The RFID tag allows for the exchange of product information such as lot number, expiration, concentration, and identification of the syringe as being “unused” prior to use and “used” after product administration.
- Do not operate any part of the Optivantage Injector System and RFID-tagged syringes within 6 inches (15 cm) of a pacemaker and/or defibrillator.
- If the RFID tag is damaged or otherwise non-functional, the Optiray syringe with the non-functional RFID tag may still be used; however, no data will be transferred to the injector.
2.3 Intra-Arterial Procedures In Adults
Cerebral Arteriography
Use Optiray 240, Optiray 300 or Optiray 320. The recommended dose for visualization of cerebral arteries is shown below (may repeat as necessary):
Diagnostic area | Dose | Maximum Cumulative Dose |
carotid or vertebral arteries | 2 to 12 mL | 200 mL |
aortic arch injection | 20 to 50 mL | 200 mL |
2.4 Intravenous Procedures In Adults
- Computed Tomography
- Scan immediately after completion of the intravenous administration.
- Scanning interval will vary with indication and target organ.
- Venography
- Intravenous Urography
- Intravenous Digital Subtraction Angiography (IV-DSA)
- Central catheter injections are usually made at a rate of between 10 and 30 mL/second.
- Peripheral injections are usually made at a rate of between 12 and 20 mL/second.
Use Optiray 240, Optiray 300, Optiray 320 or Optiray 350 for head and body imaging.
Head Imaging
The recommended dosing is shown below:
Infusion | |
Optiray 240 | 100 to 250 mL |
Optiray 300 | 50 to 150 mL |
Optiray 320 | 50 to 150 mL |
Optiray 350 | 50 to 150 mL |
Body Imaging
Optiray may be administered by bolus injection, by rapid infusion, or by a combination of both. The recommended dosing is shown below:
Bolus Injection | Infusion | |
Optiray 240 | 35 to 100 mL | 70 to 250 mL |
Optiray 300 | 25 to 75 mL | 50 to 150 mL |
Optiray 320 | 25 to 75 mL | 50 to 150 mL |
Optiray 350 | 25 to 75 mL | 50 to 150 mL |
Use Optiray 240, Optiray 300, Optiray 320 or Optiray 350. The recommended dose is 50 to 100 mL per extremity; with a maximum cumulative dose of 250 mL.
Use Optiray 350, Optiray 320, Optiray 300 or Optiray 240. The recommended dose is shown below:
Usual Dose | High Dose Urography | Maximum Dose | |
Optiray 240 | 75 to 100 mL | 2 mL/kg | 200 mL |
Optiray 300 | 50 to 75 mL | 1.6 mL/kg | 150 mL |
Optiray 320 | 50 to 75 mL | 1.5 to 2 mL/kg | 150 mL |
Optiray 350 | 50 to 75 mL | 1.4 mL/kg | 140 mL |
Use Optiray 350. The recommended dose range per injection is 30 to 50 mL; may repeat as necessary with a maximum cumulative dose of 250 mL.
Injection rates will vary depending on the site of catheter placement and vessel size.
2.5 Pediatric Dosing
Intra-arterial Procedures
- Angiocardiography
- Computed Tomography
- Intravenous Urography
Use Optiray 350 or Optiray 320. The recommended single ventricular dose is 1.25 mL/kg (range 1 mL/kg to 1.5 mL/kg). The maximum cumulative dose is 5 mL/kg up to a maximum total volume of 250 mL.
Intravenous Procedures
Use Optiray 320.
Head and Body Imaging
The recommended dose in pediatric patients is 1.5 mL/kg to 2 mL/kg (range 1 mL/kg to 3 mL/kg).
Use Optiray 320. The recommended dose for pediatric patients is 1 mL/kg to 1.5 mL/kg (range 0.5 mL/kg to 3 mL/kg); with a maximum cumulative dose not exceeding 3 mL/kg.
3 Dosage Forms And Strengths
Injection: clear, colorless to pale yellow solutions containing no undissolved solids, available in the following strengths and single-dose containers:
Imaging Product | mg of ioversol per mL | mg of organically boundiodine per mL | Presentations | |||
Vials | Bottles | Hand-held syringes | Power injector syringes | |||
OPTIRAY 240 (Ioversol 51%) | 509 | 240 | No | Yes | No | Yes |
OPTIRAY 300 (Ioversol 64%) | 636 | 300 | No | Yes | Yes | Yes |
OPTIRAY 320 (Ioversol 68%) | 678 | 320 | Yes | Yes | Yes | Yes |
OPTIRAY 350 (Ioversol 74%) | 741 | 350 | No | Yes | Yes | Yes |
4 Contraindications
Symptomatic Hyperthyroidism
5.1 Risks Associated With Inadvertent Intrathecal Administration
Optiray is indicated for intravascular use only [see Dosage and Administration (2.1)]. Inadvertent intrathecal administration can cause death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema.
5.2 Hypersensitivity Reactions
Optiray can cause life-threatening or fatal hypersensitivity reactions including anaphylaxis and anaphylactic shock. Manifestations include respiratory arrest, laryngospasm, bronchospasm, angioedema, and shock. Most severe reactions develop shortly after the start of the injection (e.g. within 1 to 3 minutes), but delayed reactions may occur. There is an increased risk in patients with a history of a previous reaction to contrast agent, and known allergies (i.e., bronchial asthma, drug, or food allergies), and other hypersensitivities. Premedication with antihistamines or corticosteroids to avoid or minimize possible allergic reactions does not prevent serious life-threatening reactions, but may reduce both their incidence and severity.
Obtain a history of allergy, hypersensitivity, or prior hypersensitivity reactions to iodinated contrast agents. Always have emergency resuscitation equipment and trained personnel available and monitor all patients for hypersensitivity reactions.
5.3 Contrast Induced Acute Kidney Injury
Acute kidney injury, including renal failure, may occur after Optiray administration. Risk factors include: pre-existing renal impairment, dehydration, diabetes mellitus, congestive heart failure, advanced vascular disease, elderly age, concomitant use of nephrotoxic or diuretic medications, multiple myeloma / paraproteinaceous diseases, repetitive and/or large doses of an iodinated contrast agent.
Use the lowest necessary dose of Optiray in patients with renal impairment. Adequately hydrate patients prior to and following Optiray administration. Do not use laxatives, diuretics, or preparatory dehydration prior to Optiray administration.
5.4 Cardiovascular Adverse Reactions
Optiray increases the circulatory osmotic load and may induce acute or delayed hemodynamic disturbances in patients with congestive heart failure, severely impaired renal function, combined renal and hepatic disease, combined renal and cardiac disease, particularly when repetitive or large doses are administered.
Life-threatening or fatal cardiovascular reactions have occurred with the use of Optiray, including cardiac arrest, hypotensive collapse, and shock. Most deaths occur within 10 minutes of injection; with cardiovascular disease as the main underlying factor. Cardiac decompensation, serious arrhythmias, and myocardial ischemia or infarction can occur during coronary arteriography and ventriculography.
Based upon literature reports, deaths from the administration of iodinated contrast agents range from 6.6 per 1 million (0.00066 percent) to 1 in 10,000 patients (0.01 percent). Use the lowest necessary dose of Optiray in patients with congestive heart failure and always have emergency resuscitation equipment and trained personnel available. Monitor all patients for severe cardiovascular reactions.
5.5 Thromboembolic Events
Angiocardiography
Serious, fatal, thromboembolic events causing myocardial infarction and stroke can occur during angiographic procedures with Optiray. During these procedures, increased thrombosis and activation of the complement system occurs. Risk factors for thromboembolic events include: length of procedure, catheter and syringe material, underlying disease state, and concomitant medications.
To minimize thromboembolic events use meticulous angiographic technique. Avoid blood remaining in contact with syringes containing Optiray, which increases the risk of clotting. Avoid angiocardiography in patients with homocystinuria because of the risk of inducing thrombosis and embolism [see Clinical Pharmacology (12.2)].
5.6 Extravasation And Injection Site Reactions
Extravasation can occur with Optiray administration, particularly in patients with severe arterial or venous disease and can be associated with pain, hemorrhage and necrosis. Ensure intravascular placement of catheters prior to injection. Monitor patients for extravasation and advise patients to seek medical care for progression of symptoms.
5.7 Thyroid Storm In Patients With Hyperthyroidism
Optiray is contraindicated in patients with symptomatic hyperthyroidism [see Contraindications (4)]. Thyroid storm has occurred following the intravascular use of iodinated radiopaque agents in patients with hyperthyroidism or with an autonomously functioning thyroid nodule. Evaluate the risk in such patients before use of Optiray.
5.8 Hypertensive Crisis In Patients With Pheochromocytoma
Hypertensive crisis has occurred after the use of iodinated radiopaque contrast agents in patient with pheochromocytoma. Closely monitor patients when administering Optiray if pheochromocytoma or catecholamine-secreting paraganglioma is suspected. Inject the minimum amount of Optiray necessary and have measures for treatment of hypertensive crisis readily available.
5.9 Sickle Cell Crisis In Patients With Sickle Cell Disease
Iodinated contrast agents may promote sickling in individuals who are homozygous for sickle cell disease. Hydrate patients prior to and following Optiray administration, use Optiray only if the necessary imaging information cannot be obtained with alternative imaging modalities, and inject the minimum amount necessary.
5.10 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions (SCAR) may develop from 1 hour to several weeks after intravascular contrast agent administration. These reactions include Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS). Reaction severity may increase and time to onset may decrease with repeat administration of a contrast agent; prophylactic medications may not prevent or mitigate severe cutaneous adverse reactions. Avoid administering Optiray to patients with a history of a severe cutaneous adverse reaction to Optiray.
6 Adverse Reactions
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risks Associated with Inadvertent Intrathecal Administration [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
- Contrast Induced Acute Kidney Injury [see Warnings and Precautions (5.3)]
- Cardiovascular Adverse Reactions [see Warnings and Precautions (5.4)]
- Thromboembolic Events [see Warnings and Precautions (5.5)]
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.10)]
6.1 Clinical Studies Experience
Adult Patients
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following listing shows adverse reactions based upon clinical trials with Optiray (ioversol) in 4,187 patients. Adverse reactions are listed by organ system according to clinical importance. More severe reactions are listed before others in a system regardless of incidence. The most common reaction is nausea, occurring at a rate of 1 percent.
Cardiac disorders
Cardiac arrest, myocardial infarction, arrhythmia, atrioventricular block complete, atrioventricular block, nodal rhythm, bradycardia, angina pectoris, palpitations
Ear and labyrinth disorders
Vertigo, tinnitus
Eye disorders
Vision blurred, periorbital edema, conjunctivitis
Gastrointestinal disorders
Nausea, vomiting, abdominal pain, dysphagia, dry mouth
General disorders and administration site conditions
Chest pain, pain, injection site pain, injection site hematoma, extravasation, pyrexia, swelling, asthenia, malaise, fatigue, chills
Infections and infestations
Rhinitis
Injury, poisoning, and procedural complications
Heart injury, vascular pseudoaneurysm
Investigations
Electrocardiogram ST segment depression, blood pressure decreased
Metabolism and nutrition disorders
Acidosis
Musculoskeletal and connective tissue disorders
Muscular weakness, muscle spasms, back pain
Nervous system disorders
Cerebral infarction, aphasia, tremor, dizziness, presyncope, headache, paraesthesia, dysgeusia
Psychiatric disorders
Hallucination, visual hallucination, disorientation, anxiety
Renal and urinary disorders
Urinary retention, renal pain, polyuria
Respiratory, thoracic, and mediastinal disorders
Laryngeal edema, hypoxia, pulmonary edema, dyspnea, hyperventilation, cough, sneezing, nasal congestion
Skin and subcutaneous tissue disorders
Urticaria, rash, pruritus, swelling face, hyperhidrosis, erythema
Vascular disorders
Hypertension, hypotension, arterial spasm, vasospasm, vasodilation, flushing
Pediatric Patients
In clinical studies involving 311 patients for pediatric angiocardiography, contrast enhanced computed tomographic imaging of the head and body, and intravenous excretory urography; 6% of patients reported an adverse reaction, with the most common adverse reactions being nausea and fever. Adverse reactions reported were similar in quality and frequency to the adverse events reported by adults.
6.2 Postmarketing Experience
The following additional adverse drug reactions have been reported during post-approval use of Optiray. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate frequency.
Cardiac disorders: coronary artery spasm, cyanosis, arrhythmia (ventricular fibrillation, tachycardia, extrasystole), ECG abnormal.
Endocrine disorders: thyroid function tests indicative of hypothyroidism or transient thyroid suppression have been uncommonly reported following iodinated contrast media administration to adult and pediatric patients, including infants, some patients were treated for hypothyroidism.
Eye disorders: temporary blindness, conjunctivitis (including eye irritation, ocular hyperemia, watery eyes).
Gastrointestinal disorders: tongue edema, salivary hypersecretion.
General disorders and administration site conditions: injection site reactions including pain, hemorrhage, and necrosis especially after extravasation [see Warnings and Precautions (5.6)], face edema, feeling hot.
Immune system disorders: hypersensitivity reactions including fatal anaphylactic shock.
Nervous system disorders: seizure, loss of consciousness, somnolence, hypoesthesia, dyskinesia, amnesia.
Respiratory disorders: Respiratory arrest, asthma, bronchospasm, laryngeal spasm and obstruction, throat irritation, dysphonia.
Skin and subcutaneous tissue disorders: Reactions range from mild (e.g. rash, erythema, pruritus, urticaria, and skin discoloration) to severe: [e.g. Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN)], acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS).
Vascular Disorders: phlebitis, thrombosis.
7 Drug Interactions
7.1 Drug-Drug Interactions
- Metformin
- Radioactive Iodine
- Oral Cholecystographic Contrast Agents
In patients with renal impairment, metformin can cause lactic acidosis. Iodinated contrast agents appear to increase the risk of metformin induced lactic acidosis, possibly as a result of worsening renal function. Stop metformin at the time of, or prior to, Optiray administration in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast agents. Re-evaluate eGFR 48 hours after the imaging procedure, and reinstitute only after renal function is stable.
Administration of iodinated contrast agents may interfere with thyroid uptake of radioactive iodine (I 131) and decrease therapeutic efficacy in patients with carcinoma of the thyroid. The decrease in efficacy lasts for 6-8 weeks.
Renal toxicity has been reported in patients with liver impairment who were given oral cholecystographic agents followed by intravascular contrast agents. Administration of Optiray should be postponed in patients who have recently received a cholecystographic contrast agent.
7.2 Drug/Laboratory Test Interactions
- Protein-Bound Iodine, Radioactive Iodine Determinations
The results of protein bound iodine and radioactive iodine uptake studies, which depend on iodine estimation, will not accurately reflect thyroid function for up to 16 days following administration of iodinated contrast agent. However, thyroid function tests that do not depend on iodine estimations, e.g., T3 resin uptake and total or free thyroxine (T4) assays are not affected.
8.1 Pregnancy
Risk Summary
Postmarketing data with Optiray use in pregnant women are insufficient to determine if there is a risk of drug-associated adverse developmental outcomes. Ioversol crosses the placenta and reaches fetal tissues in small amounts [see Data]. In animal reproduction studies, no adverse developmental effects were observed following daily intravenous administrations of ioversol to pregnant rats (from Gestation Day 7 to 17) and rabbits (Gestation Day 6 to 18) at doses 0.35 and 0.71 times, respectively, the maximum recommended human dose.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of major birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Human Data
Literature reports show that ioversol crosses the placenta and is visualized in the digestive tract of exposed infants after birth.
Animal Data
Developmental toxicity studies were conducted with ioversol given intravenously at doses of 0, 0.2, 0.8, and 3.2 g iodine/kg/day from Gestation Day 7 to 17 and 6 to 18, in rats and rabbits, respectively. No adverse effects on embryo-fetal development were observed in either species at the maximum dose tested (3.2 g iodine/kg/day). Maternal toxicity was observed in rabbits at 0.8 and 3.2 g iodine/kg/day.
8.2 Lactation
Risk Summary
There is no information about the presence of ioversol in human or animal milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. However, iodinated contrast agents are excreted unchanged in human milk in very low amounts with poor absorption from the gastrointestinal tract of the breastfed infant. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Optiray and any potential adverse effects on the breastfed infant from Optiray or from the underlying maternal condition.
Clinical Considerations
Interruption of breastfeeding after exposure to iodinated contrast agents is not necessary because the potential exposure of the breastfed infant to iodine is small. However, a lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk for 8 hours (approximately 5 elimination half-lives) after Optiray administration in order to minimize drug exposure to a breast fed infant.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have been established for the use of Optiray 350 and Optiray 320 in angiocardiography; and for Optiray 320 in computed tomographic imaging of the head and body, and intravenous excretory urography. Use of Optiray 350 and Optiray 320 in these age groups is based on controlled clinical trials involving 159 patients for pediatric angiocardiography; computed tomographic imaging of the head and body, and intravenous excretory urography. In general, the types of adverse reactions reported are similar to those of adults [see Adverse Reactions (6.1)].
Safety and effectiveness of Optiray 240/300 have not been established in pediatric patients.
Pediatric patients at higher risk of experiencing adverse reactions to Optiray include patients with: asthma, sensitivity to medication and/or allergens, congestive heart failure, serum creatinine greater than 1.5 mg/dL, or age less than 12 months. Thyroid function tests indicative of hypothyroidism or transient thyroid suppression have been uncommonly reported following iodinated contrast media administration in pediatric patients, including infants. Some patients were treated for hypothyroidism [See Adverse Reactions (6.2)].
8.5 Geriatric Use
Ioversol is nearly completely excreted as parent drug by the kidney, and the risk of adverse reactions to Optiray may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, dose selection should be cautious usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment
Ioversol is nearly completely excreted as parent drug by the kidney and renal impairment is expected to reduce the rate of elimination. Ioversol can be removed by dialysis.
10 Overdosage
The adverse effects of overdosage are life-threatening and affect mainly the pulmonary and cardiovascular system. Treatment of an overdosage is directed toward the support of all vital functions and prompt institution of symptomatic therapy.
Ioversol does not bind to plasma or serum protein and is, therefore, dialyzable.
11 Description
11.1 Chemical Characteristics
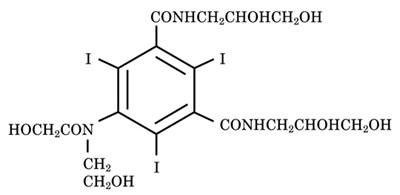
Optiray (ioversol injection) is a non-ionic radiographic contrast agent. Optiray formulations are sterile, nonpyrogenic, aqueous solutions intended for intravascular use. Ioversol is designated chemically as N,N'-Bis (2,3-dihydroxypropyl)-5-[N-(2-hydroxyethyl) -glycolamido] -2,4,6-triiodoisophthalamide. The molecular weight of ioversol is 807.11 and the organically bound iodine content is 47.2%.
The structural formula of ioversol is as follows:
Optiray is available in four strengths:
- Optiray 240 (ioversol injection 51%): Each mL contains 240 mg organically bound iodine, 509 mg ioversol, 3.6 mg tromethamine, 0.2 mg of edetate calcium disodium.
- Optiray 300 (ioversol injection 64%): Each mL contains 300 mg organically bound iodine, 636 mg ioversol, 3.6 mg, tromethamine, 0.2 mg edetate calcium disodium.
- Optiray 320 (ioversol injection 68%): Each mL contains 320 mg organically bound iodine, 678 mg of ioversol, 3.6 mg tromethamine, 0.2 mg edetate calcium disodium.
- Optiray 350 (ioversol injection 74%): Each mL contains 350 mg organically bound iodine, 741 mg ioversol, 3.6 mg tromethamine, 0.2 mg edetate calcium disodium.
The pH of the Optiray formulations is adjusted to 6.0 to 7.4 with hydrochloric acid or sodium hydroxide. All solutions are sterilized by autoclaving and contain no preservatives. Ioversol does not dissociate in solution.
11.2 Physical Characteristics
Some physical and chemical properties of these formulations are listed below:
Optiray | Optiray | Optiray | Optiray | |
Ioversol content (mg/mL) | 509 | 636 | 678 | 741 |
Iodine content | 240 | 300 | 320 | 350 |
Osmolality (mOsm/kg water) | 502 | 651 | 702 | 792 |
Viscosity (cps) | ||||
at 25°C | 4.6 | 8.2 | 9.9 | 14.3 |
at 37°C | 3.0 | 5.5 | 5.8 | 9.0 |
Specific Gravity at 37°C | 1.281 | 1.352 | 1.371 | 1.405 |
The Optiray formulations are clear, colorless to pale yellow solutions containing no undissolved solids. Crystallization does not occur at room temperature. Optiray solutions have osmolalities 1.8 to 2.8 times that of plasma (285 mOsm/kg water) as shown in the above table and are hypertonic under conditions of use.
12.1 Mechanism Of Action
Intravascular injection of ioversol opacifies vessels in the path of the flow of the contrast medium, permitting visualization of the internal structures until significant hemodilution occurs.
In imaging of the body, iodinated contrast agents diffuse from the vascular into the extravascular space. In normal brain with an intact blood-brain barrier, contrast does not diffuse into the extravascular space. In patients with a disrupted blood-brain barrier, contrast agent accumulates in the interstitial space in the region of disruption.
12.2 Pharmacodynamics
Following administration of Optiray, the degree of enhancement is directly related to the iodine content in an administered dose. Peak iodine plasma levels occur immediately following rapid injection. The time to maximum contrast enhancement can vary, depending on the organ, from the time that peak blood iodine concentrations are reached to one hour after intravenous bolus administration. When a delay between peak blood iodine concentrations and peak contrast is present, it suggests that radiographic contrast enhancement is at least in part dependent on the accumulation of iodine-containing medium within the lesion and outside the blood pool.
For angiography, contrast enhancement is greatest immediately (15 seconds to 120 seconds) after rapid injection. Iodinated contrast agents may be visualized in the renal parenchyma within 30-60 seconds following rapid intravenous injection. Opacification of the calyces and pelves in patients with normal renal function becomes apparent within 1-3 minutes, with optimum contrast occurring within 5-15 minutes.
12.3 Pharmacokinetics
In 12 healthy volunteers (6 receiving 50 mL and 6 receiving 150 mL of Optiray 320), elimination half-life was 1.5 hours for both doses.
Distribution
In an in vitro human plasma study, ioversol did not bind to protein. The volume of distribution in adults was 0.26 L/kg body weight, consistent with distribution to the extracellular space.
Elimination
Metabolism
Ioversol does not undergo significant metabolism, deiodination or biotransformation.
Excretion
Greater than 95% of the administered dose was excreted in urine within the first 24 hours, with the peak urine concentration occurring in the first two hours after administration.
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
No long term animal studies have been performed to evaluate carcinogenic potential. Nonclinical studies show that ioversol is not mutagenic and does not affect fertility.
13.2 Animal Toxicology And/Or Pharmacology
Animal studies indicate that ioversol does not cross the blood-brain barrier.
16 How Supplied/Storage And Handling
16.1 How Supplied
Optiray is a clear, colorless to pale yellow, sterile, pyrogen-free, aqueous solution available in four strengths. The products are supplied in containers from which the air has been displaced by nitrogen. Optiray is supplied in the following single-dose configurations:
Optiray 350 | NDC Number |
Glass | |
25x50 mL bottles | 0019-1333-06 |
12x100 mL bottles | 0019-1333-11 |
12x150 mL bottles | 0019-1333-16 |
12x200 mL fill/250 mL bottles | 0019-1333-21 |
Plastic | |
20x50 mL hand held syringes | 0019-1333-78 |
20x50 mL fill/125 mL power injector syringes | 0019-1333-52 |
20x75 mL fill/125 mL power injector syringes | 0019-1333-95 |
20x100 mL fill/125 mL power injector syringes | 0019-1333-90 |
20x125 mL power injector syringes | 0019-1333-87 |
RFID-Tagged Syringes* | |
20x50 mL fill/125 mL power injector syringes | 0019-1333-55 |
20x100 mL fill/125 mL power injector syringes | 0019-1333-00 |
20x125 mL power injector syringes | 0019-1333-27 |
Optiray 320 | |
Glass | |
25x20 mL vials | 0019-1323-02 |
25x30 mL vials | 0019-1323-04 |
25x50 mL bottles | 0019-1323-06 |
12x100 mL bottles | 0019-1323-11 |
12x150 mL bottles | 0019-1323-16 |
12x200 mL fill/250 mL bottles | 0019-1323-21 |
Plastic | |
20x50 mL hand held syringes | 0019-1323-78 |
20x50 mL fill/125 mL power injector syringes | 0019-1323-52 |
20x75 mL fill/125 mL power injector syringes | 0019-1323-95 |
20x100 mL fill/125 mL power injector syringes | 0019-1323-90 |
20x125 mL power injector syringes | 0019-1323-87 |
RFID-Tagged Syringes* | |
20x75 mL fill/125 mL power injector syringes | 0019-1323-85 |
20x100 mL fill/125 mL power injector syringes | 0019-1323-00 |
20x125 mL power injector syringes | 0019-1323-27 |
Optiray 300 | |
Glass | |
25x50 mL bottles | 0019-1332-06 |
12x100 mL bottles | 0019-1332-11 |
12x150 mL bottles | 0019-1332-16 |
12x200 mL fill/250 mL bottles | 0019-1332-21 |
Plastic | |
20x50 mL hand held syringes | 0019-1332-78 |
20x100 mL fill/125 mL power injector syringes | 0019-1332-90 |
RFID-Tagged Syringes* | |
20x100 mL fill/125 mL power injector syringes | 0019-1332-00 |
Optiray 240 | |
Glass | |
25x50 mL bottles | 0019-1324-06 |
12x100 mL bottles | 0019-1324-11 |
12x200 mL fill/250 mL bottles | 0019-1324-21 |
Plastic | |
20x125 mL power injector syringes | 0019-1324-87 |
*Radio Frequency Identification (RFID) Technology
RFID-Tagged Syringe Description
This information is for UltrajectTM syringes containing Optiray that have been labeled with a Radio Frequency Identification (RFID) tag. When used with an RFID-enabled OptivantageTM injector, this tag allows for the exchange of product information such as lot number, expiration, concentration, and identification of the syringe as being “unused” prior to use and “used” after product administrations. Patient information is not used in any form with this RFID technology. Optiray product quality is not influenced by the use of this RFID tag.
RFID-Tagged Syringe Directions for Use
For the RFID Technology to function, the syringe must be used with an Optivantage Injector with RFID technology [see Dosage and Administration (2.2)]. Function of the RFID technology is not dependent on syringe orientation as it is placed in the injector. Instructions for use of injector are provided on the injector interface screen and operator’s manual.
If the RFID tag is damaged or otherwise non-functional, the injector will notify the user. Should this occur the Optiray syringe with the non-functional RFID tag may still be used but no data will be transferred to the injector.
Regarding interference with medical devices, the RFID tag and injector system meet the IEC 60601-1-2 requirements for emission and immunity standards for medical devices. Follow all manufacturers’ guidelines and do not operate any part of the Optivantage Injector System and RFID-tagged syringes within 6 inches (15 cm) of a pacemaker and/or defibrillator.
16.2 Storage
- Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
- Protect from strong daylight or direct exposure to the sun.
- Store up to 40°C (104°F) for up to one month in a contrast media warmer utilizing circulating warm air.
- May store Optiray for periods longer than one month.
- Store Optiray RFID-tagged syringes at the same conditions listed for the drug product.
- Discard Optiray syringes, glass bottles, and their contents if they are frozen or if crystallization occurs.
16.3 Handling
- Do not re-autoclave plastic container because of possible damage to syringe.
- RFID-tagged syringes require no special handling.
17 Patient Counseling Information
Hypersensitivity Reactions
Advise the patient concerning the risk of hypersensitivity reactions that can occur both during and after Optiray administration. Advise the patient to report any signs or symptoms of hypersensitivity reactions during the procedure and to seek medical attention for signs or symptoms experienced after discharge [see Warnings and Precautions (5.2)].
Advise patients to inform their physician if they develop a rash after receiving Optiray [see Warnings and Precautions (5.10)].
Contrast Induced Acute Kidney Injury
Advise the patient concerning appropriate hydration to decrease the risk of contrast induced kidney injury [see Warnings and Precautions (5.3)].
Extravasation
If extravasation occurs during injection, advise patients to seek medical care for progression of symptoms [see Warnings and Precautions (5.6)].
Package Label - Principal Display Panel - Optiray 350, 125 Ml Syringe Label
For Intravascular Use
Sterile Solution
125 mL
NDC 0019-1333-87
Optiray™ 350
Ioversol Injection 74%
350 mg/mL Organically Bound Iodine
NOT FOR INTRATHECAL USE
Rx only
Medication and fluid pathway are sterile. Outside of syringe is not sterile.
Single dose syringe • Discard unused portion and syringe.
Protect from light • Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Each mL contains 741 mg ioversol, 3.6 mg tromethamine as a buffer and 0.2 mg edetate calcium disodium as a stabilizer. The pH is adjusted with hydrochloric acid or sodium hydroxide.
Discard contents if syringe is frozen, if crystallization occurs, syringe seal is broken or if leaking is observed.
Usual Dosage: See Package Insert for indications, dosage and dispensing information.
Manufactured by:
Liebel-Flarsheim Company LLC
Raleigh, NC 27616
Made in USA
15570916
Package Label - Principal Display Panel - Optiray 320, 125 Ml Syringe Label
For Intravascular Use
Sterile Solution
125 mL
NDC 0019-1323-87
Optiray™ 320
Ioversol Injection 68%
320 mg/mL Organically Bound Iodine
NOT FOR INTRATHECAL USE
Rx only
Medication and fluid pathway are sterile. Outside of syringe is not sterile.
Single dose syringe • Discard unused portion and syringe.
Protect from light • Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Each mL contains 678 mg ioversol, 3.6 mg tromethamine as a buffer and 0.2 mg edetate calcium disodium as a stabilizer. The pH is adjusted with hydrochloric acid or sodium hydroxide.
Discard contents if syringe is frozen, if crystallization occurs, syringe seal is broken or if leaking is observed.
Usual Dosage: See Package Insert for indications, dosage and dispensing information.
Manufactured by:
Liebel-Flarsheim Company LLC
Raleigh, NC 27616
Made in USA
15510916
Package Label - Principal Display Panel - Optiray 300, 100 Ml Syringe Label
For Intravascular Use
Sterile Solution
100 mL
NDC 0019-1332-90
Optiray™ 300
Ioversol Injection 64%
300 mg/mL Organically Bound Iodine
NOT FOR INTRATHECAL USE
Rx only
Medication and fluid pathway are sterile. Outside of syringe is not sterile.
Single dose syringe • Discard unused portion and syringe.
Protect from light • Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Each mL contains 636 mg ioversol, 3.6 mg tromethamine as a buffer and 0.2 mg edetate calcium disodium as a stabilizer. The pH is adjusted with hydrochloric acid or sodium hydroxide.
Discard contents if syringe is frozen, if crystallization occurs, syringe seal is broken or if leaking is observed.
Usual Dosage: See Package Insert for indications, dosage and dispensing information.
Manufactured by:
Liebel-Flarsheim Company LLC
Raleigh, NC 27616
Made in USA
15440916
Package Label - Principal Display Panel - Optiray 240, 125 Ml Syringe Label
For Intravascular Use
Sterile Solution
125 mL
NDC 0019-1324-87
Optiray™ 240
Ioversol Injection 51%
240 mg/mL Organically Bound Iodine
NOT FOR INTRATHECAL USE
Rx only
Medication and fluid pathway are sterile. Outside of syringe is not sterile.
Single dose syringe • Discard unused portion and syringe.
Protect from light • Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Each mL contains 509 mg ioversol, 3.6 mg tromethamine as a buffer and 0.2 mg edetate calcium disodium as a stabilizer. The pH is adjusted with hydrochloric acid or sodium hydroxide.
Discard contents if syringe is frozen, if crystallization occurs, syringe seal is broken or if leaking is observed.
Usual Dosage: See Package Insert for indications, dosage and dispensing information.
Manufactured by:
Liebel-Flarsheim Company LLC
Raleigh, NC 27616
Made in USA
15410916
* Please review the disclaimer below.