Product Images Zavzpret
View Photos of Packaging, Labels & Appearance
- Chemical Structure - zavegepant 01
- Figure 1 - zavegepant 02
- Figure 2 - zavegepant 03
- Figure 3 - zavegepant 04
- Figure 4 - zavegepant 05
- Logo - zavegepant 06
- Logo - zavegepant 07
- Figure A - zavegepant 08
- Figure B - zavegepant 09
- Figure C - zavegepant 10
- Figure D - zavegepant 11
- Figure E - zavegepant 12
- Figure F - zavegepant 13
- Figure G - zavegepant 14
- Figure H - zavegepant 15
- Figure I - zavegepant 16
- Figure J - zavegepant 17
- Figure K - zavegepant 18
- Figure L - zavegepant 19
- Logo - zavegepant 20
- PRINCIPAL DISPLAY PANEL – Nasal Spray Device Label Front - zavzpret 07
- PRINCIPAL DISPLAY PANEL – Nasal Spray Device Label Back - zavzpret 08
- PRINCIPAL DISPLAY PANEL – Nasal Spray Blister Foil Label - zavzpret 09
- PRINCIPAL DISPLAY PANEL – Nasal Spray Carton - zavzpret 10
Product Label Images
The following 24 images provide visual information about the product associated with Zavzpret NDC 0069-3500 by Pfizer Laboratories Div Pfizer Inc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Logo - zavegepant 06

Stfier is a product that is distributed by Pfizer Labs, a division of Pfizer Inc. based in New York. It is not possible to provide any further information based on the limited text provided.*
Logo - zavegepant 07

Stfier is distributed by Pfizer Labs, a division of Pfizer Inc. located in New York, NY 10001.*
Figure K - zavegepant 18
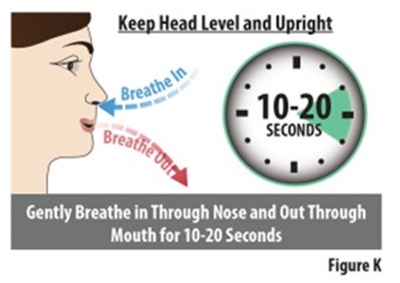
The text describes a breathing exercise to be performed by inhaling through the nose and exhaling through the mouth for a duration of 10-20 seconds. It is accompanied by a figure labeled K.*
Logo - zavegepant 20

Stfier is a product distributed by Pfizer Labs, which is a division of Pfizer Inc. based in New York, NY 10001.*
PRINCIPAL DISPLAY PANEL – Nasal Spray Device Label Front - zavzpret 07

Zavegepant nasal spray is a medication meant for intranasal use only with a strength of 10mg. The product is identified by the NDC number 0069-3500-01. No other information is available due to the limited and unclear text obtained by .*
PRINCIPAL DISPLAY PANEL – Nasal Spray Blister Foil Label - zavzpret 09

This is a medication description for Zavegepant nasal spray, with the NDC code 0069-3500-01. Each unit-dose device contains 10 mg of the medication, and should only be used intranasally. The recommended dosage can be found in the prescribing information, and the device should be kept in the sealed blister package until use. The medication should be stored at 20°C to 25°C (68°F to 77°F), with excursions permitted between 15°C to 30°C (59°F to 86°F), and should not be frozen. The spray should not be tested or primed before use, and the device should be kept out of reach of children. This medication is distributed by Pfizer Inc. from New York, NY.*
PRINCIPAL DISPLAY PANEL – Nasal Spray Carton - zavzpret 10

This appears to be a description of a nasal spray, containing 10mg of a medication. The name of the medication is not available as it was not provided in the text. The text "Zavzpret:z T ." and "buw oy Aesds jeseu" are not readable and may be errors.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.
















