Product Images Accupril
View Photos of Packaging, Labels & Appearance
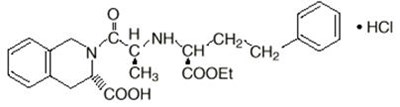
- Chemical Structure - accupril 01
- Logo - accupril 02
- PRINCIPAL DISPLAY PANEL - 5 mg Tablet Bottle Label - accupril 03
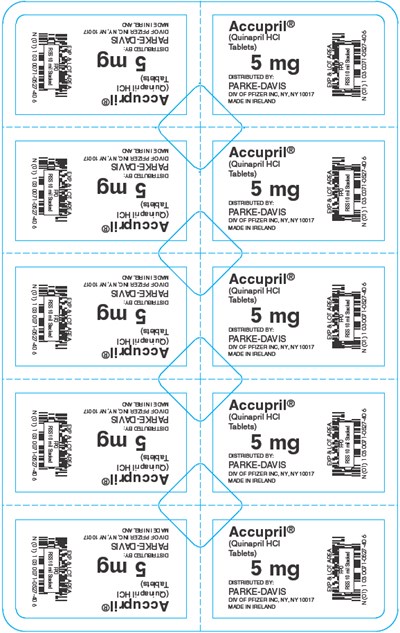
- PRINCIPAL DISPLAY PANEL - 5 mg Blister Pack - accupril 04
- PRINCIPAL DISPLAY PANEL - 5 mg Carton - accupril 05
- PRINCIPAL DISPLAY PANEL - 10 mg Tablet Bottle Label - accupril 06
- PRINCIPAL DISPLAY PANEL - 10 mg Blister Pack - accupril 07
- PRINCIPAL DISPLAY PANEL - 10 mg Carton - accupril 08
- PRINCIPAL DISPLAY PANEL - 20 mg Tablet Bottle Label - accupril 09
- PRINCIPAL DISPLAY PANEL - 20 mg Blister Pack - accupril 10
- PRINCIPAL DISPLAY PANEL - 20 mg Carton - accupril 11
- PRINCIPAL DISPLAY PANEL - 40 mg Tablet Bottle Label - accupril 12
- accupril 13
- accupril 14
- accupril 15
- accupril 16
Product Label Images
The following 16 images provide visual information about the product associated with Accupril NDC 0071-0532 by Parke-davis Div Of Pfizer Inc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Logo - accupril 02

This is a statement about the distribution of a product by Parke-Davis, which is a division of Pfizer Inc, located in New York with the ZIP code 10017.*
PRINCIPAL DISPLAY PANEL - 5 mg Carton - accupril 05

This is a description of Accupril® (Quinapril HCI Tablets), a medication used for in-institution use only. Each tablet contains quinapril HCI, equivalent to 5mg quinapri. The drug comes in a box with 100 tablets, and each tablet is individually packaged as a unit dose. The product is distributed by Parke-Davis, a division of Pfizer Inc in New York, and made in Ireland. The box and individual tablets should be stored at a controlled room temperature of 15°-30°C (59°-86°F) and protected from light. The dosage and use of the medication are specified in the accompanying prescribing information.*
PRINCIPAL DISPLAY PANEL - 10 mg Tablet Bottle Label - accupril 06

This is a pharmaceutical product information of Accupril (Quinapril HCI Tablets) containing details of recommended storage conditions, dosage, and manufacturer information. The product contains 10mg quinapril HCI and is distributed by Parke-Davis, a division of Pfizer Inc. The package comes as 90 tablets and is only available on prescription. The lot number and expiration date are also provided. The product code and other details about dosage and usage can be found in the accompanying prescribing information.*
PRINCIPAL DISPLAY PANEL - 10 mg Blister Pack - accupril 07

Accupril is a medication containing Quinapril HCI used to treat high blood pressure. It is available in tablet form with a 10mg dosage strength produced by Park-Davis.*
PRINCIPAL DISPLAY PANEL - 10 mg Carton - accupril 08

This is a description of a medication called Accupril. It is a quinapril HCI tablet that is available in a unit dose of 100 tablets. The drug is meant for in-institution use only and requires a prescription. Each Accupril HCI tablet contains an equivalent of 10mg of quinapril HCI. The medication should be stored at a controlled room temperature of 15°-30°C (59°-86°F) and protected from light. The accompanying prescribing information should be consulted for dosage and use directions. The drug is manufactured in Ireland and distributed by Parke-Davis, a division of Pfizer Inc in New York, NY. The GTIN number is 00300710530409.*
PRINCIPAL DISPLAY PANEL - 20 mg Tablet Bottle Label - accupril 09

This is a medication called Quinapril HCI Tablets, which is distributed by Parke-Davis, a division of Pfizer Inc. It is recommended to store the medication in a controlled room temperature of 15°-30°C or 50°-86°F and protect it from light. Each tablet contains 20 mg of Quinapril HCI. The container follows USP guidelines. More information about the dosage and use can be found in the accompanying prescribing information. Made in Ireland, the package contains 90 tablets, and is only available through prescription. The other information seems to be a product code, UPC, and GTIN that likely identify the medication in a supply chain management system.*
PRINCIPAL DISPLAY PANEL - 20 mg Blister Pack - accupril 10

This is a list of medication for Accupril® (Quinapril HCl tablets) of 20mg manufactured by Parke-Davis. The text appears to be a repetition of the same description, with minor variations, and the rest of the information seems to be unavailable or not properly -recognized.*
PRINCIPAL DISPLAY PANEL - 20 mg Carton - accupril 11

This is a description of Accupril, a medication used in institutional settings only. The medication comes in unit dose packaging with 100 tablets per package. Each tablet contains quinapril HCI equivalent to 20 mg quinapril. The prescription information should be consulted for dosage and use instructions. Accupril should be stored at controlled room temperature between 15°C and 30°C and should be protected from light. The medication is distributed by Parke-Davis, a division of Pizer Inc, and is Made in Ireland. The product has a unique identifier, NDC 0071-0532-40, and GTIN: 00300710532603.*
PRINCIPAL DISPLAY PANEL - 40 mg Tablet Bottle Label - accupril 12

This is a prescription medicine called Accupril® containing 40 mg Quinapril HCI in each tablet. The medicine is to be stored at a temperature range of 15°C-30°C (59°F-86°F) and protected from light. It should be dispensed in a container defined by USP. The manufacturer is Parke-Davis, a division of Pfizer Inc located in NY, USA. The batch number, expiration date, and GTIN are not available. Refer to the accompanying prescription information for dosage and usage instructions.*
accupril 13

This is a prescription medicine called Accupril (quinapril) used to lower high blood pressure. The medicine is in the form of tablets and should be stored in a controlled room temperature between 15°-30°C, protected from light. The package contains 90 tablets. The prescribing information is provided along with the medicine. The manufacturer is Parke-Davis, a division of Pfizer Inc.*
accupril 14

This is a prescription drug called Accupril (quinapril) tablets. It should be stored at a controlled room temperature of 15°-30°C and protected from light. Each tablet contains 10mg of quinapril. The dosage and use should be followed based on the accompanying prescription information. The drug is distributed by Parke-Davis, a division of Pfizer Inc and made in Spain. The details of the drug's distribution such as NDC number and batch information are also provided.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.