Product Images Vivelle-dot
View Photos of Packaging, Labels & Appearance
- vivelle dot 01
- Estradiol structural formula - vivelle dot 02
- Vivelle-Dot layer image - vivelle dot 03
- Figure 1. Steady-State Estradiol Plasma Concentrations for Systems Applied to the Abdomen Nonbaseline-corrected Levels - vivelle dot 04
- Figure 2. Mean (SD) Change from Baseline in Mean Daily Number of Flushes for Vivelle 0.0375 mg Versus Placebo in a 12 week Trial - vivelle dot 05
- Figure 3. Bone Mineral Density AP Lumbar Spine Least Squares Means of Percentage Change from Baseline All Randomized Patients with at Least One Post baseline Assessment Available with Last Post baseline Observation Carried Forward - vivelle dot 06
- Figure 4. Bone Mineral Density Femoral Neck Least Squares Means of Percentage Change from Baseline All Randomized Patients with at Least One Post baseline Assessment Available with Last Post baseline Observation Carried Forward - vivelle dot 07
- Determine Your Schedule for Your Twice-a-Week Application - vivelle dot 08
- Where to Apply Vivelle-Dot® - vivelle dot 09
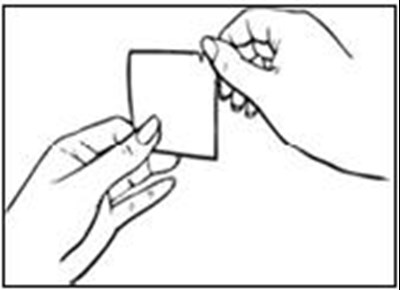
- Before You Apply Vivelle-Dot® - vivelle dot 0A
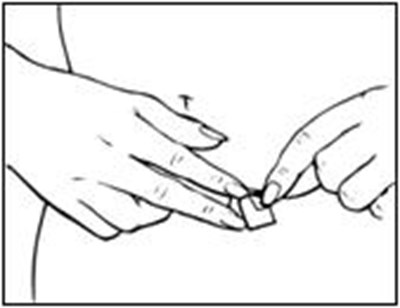
- How to Apply Vivelle-Dot® 1 - vivelle dot 0B
- How to Apply Vivelle-Dot® 2 - vivelle dot 0C
- How to Apply Vivelle-Dot® 3 - vivelle dot 0D
- How to Apply Vivelle-Dot® 4 - vivelle dot 0E
- How to Apply Vivelle-Dot® 5 - vivelle dot 0F
- How to Apply Vivelle-Dot® 6 - vivelle dot 10
- PRINCIPAL DISPLAY PANELPackage Label – 0.025 mg Rx Only NDC 0078-0365-42Vivelle-Dot® (estradiol transdermal system)Delivers 0.025 mg/dayIncludes 8 Systems - vivelle dot 11
- PRINCIPAL DISPLAY PANELPackage Label – 0.0375 mg Rx Only NDC 0078-0343-42Vivelle-Dot® (estradiol transdermal system)Delivers 0.0375 mg/dayIncludes 8 Systems - vivelle dot 12
- PRINCIPAL DISPLAY PANELPackage Label – 0.05 mg Rx Only NDC 0078-0344-42Vivelle-Dot® (estradiol transdermal system)Delivers 0.05 mg/dayIncludes 8 Systems - vivelle dot 13
- PRINCIPAL DISPLAY PANELPackage Label – 0.075 mg Rx Only NDC 0078-0345-42Vivelle-Dot® (estradiol transdermal system)Delivers 0.075 mg/dayIncludes 8 Systems - vivelle dot 14
Product Label Images
The following 20 images provide visual information about the product associated with Vivelle-dot NDC 0078-0343 by Novartis Pharmaceuticals Corporation, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1. Steady-State Estradiol Plasma Concentrations for Systems Applied to the Abdomen Nonbaseline-corrected Levels - vivelle dot 04

Figure 2. Mean (SD) Change from Baseline in Mean Daily Number of Flushes for Vivelle 0.0375 mg Versus Placebo in a 12 week Trial - vivelle dot 05

Figure 3. Bone Mineral Density AP Lumbar Spine Least Squares Means of Percentage Change from Baseline All Randomized Patients with at Least One Post baseline Assessment Available with Last Post baseline Observation Carried Forward - vivelle dot 06

This is a chart showing the percent change from baseline for different treatments over a period of 104 weeks. The treatments include Vivelle at different doses (0.1mg/day, 0.025mg/day, 0.05mg/day, and 0.0375mg/day) as well as a placebo. There are measurements taken at weeks 26, 52, 78, and 104.*
Figure 4. Bone Mineral Density Femoral Neck Least Squares Means of Percentage Change from Baseline All Randomized Patients with at Least One Post baseline Assessment Available with Last Post baseline Observation Carried Forward - vivelle dot 07
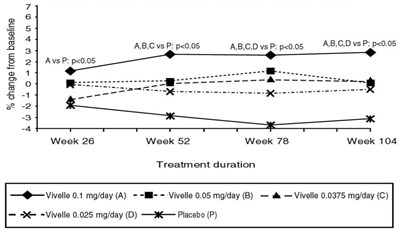
This is a statistical analysis of a study evaluating the effectiveness of different doses of Vivelle (estrogen medication) against a placebo. The text reports p-values indicating significant differences between groups (ABCD versus P, AB versus P) and percentage changes from baseline. The treatment duration and dosage for each group are also provided.*
PRINCIPAL DISPLAY PANELPackage Label – 0.025 mg Rx Only NDC 0078-0365-42Vivelle-Dot® (estradiol transdermal system)Delivers 0.025 mg/dayIncludes 8 Systems - vivelle dot 11

PRINCIPAL DISPLAY PANELPackage Label – 0.0375 mg Rx Only NDC 0078-0343-42Vivelle-Dot® (estradiol transdermal system)Delivers 0.0375 mg/dayIncludes 8 Systems - vivelle dot 12

PRINCIPAL DISPLAY PANELPackage Label – 0.05 mg Rx Only NDC 0078-0344-42Vivelle-Dot® (estradiol transdermal system)Delivers 0.05 mg/dayIncludes 8 Systems - vivelle dot 13

PRINCIPAL DISPLAY PANELPackage Label – 0.075 mg Rx Only NDC 0078-0345-42Vivelle-Dot® (estradiol transdermal system)Delivers 0.075 mg/dayIncludes 8 Systems - vivelle dot 14

* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.