FDA Label for Piqray
View Indications, Usage & Precautions
- 1 INDICATIONS AND USAGE
- 2.1 PATIENT SELECTION
- 2.2 DOSAGE AND ADMINISTRATION
- 2.3 DOSE MODIFICATIONS FOR ADVERSE REACTIONS
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5.1 SEVERE HYPERSENSITIVITY
- 5.2 SEVERE CUTANEOUS REACTIONS
- 5.3 HYPERGLYCEMIA
- 5.4 PNEUMONITIS
- 5.5 DIARRHEA
- 5.6 EMBRYO-FETAL TOXICITY
- 6 ADVERSE REACTIONS
- 6.1 CLINICAL TRIAL EXPERIENCE
- 7.1 EFFECT OF OTHER DRUGS ON PIQRAY
- 7.2 EFFECT OF PIQRAY ON OTHER DRUGS
- 8.1 PREGNANCY
- 8.2 LACTATION
- 8.3 FEMALES AND MALES OF REPRODUCTIVE POTENTIAL
- 8.4 PEDIATRIC USE
- 8.5 GERIATRIC USE
- 8.6 RENAL IMPAIRMENT
- 10 OVERDOSAGE
- 11 DESCRIPTION
- 12.1 MECHANISM OF ACTION
- 12.2 PHARMACODYNAMICS
- 12.3 PHARMACOKINETICS
- 13.1 CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
Piqray Product Label
The following document was submitted to the FDA by the labeler of this product Novartis Pharmaceuticals Corporation. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
1 Indications And Usage
PIQRAY is indicated in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer as detected by an FDA-approved test following progression on or after an endocrine-based regimen.
2.1 Patient Selection
Select patients for the treatment of HR-positive, HER2-negative advanced or metastatic breast cancer with PIQRAY, based on the presence of one or more PIK3CA mutations in tumor tissue or plasma specimens [see Clinical Studies (14)]. If no mutation is detected in a plasma specimen, test tumor tissue. Information on FDA-approved tests for the detection of PIK3CA mutations in breast cancer is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Dosage And Administration
The recommended dose of PIQRAY is 300 mg (two 150 mg film-coated tablets) taken orally, once daily, with food [see Clinical Pharmacology (12.3)].
Continue treatment until disease progression or unacceptable toxicity occurs [see Dosage and Administration (2.3)].
Patients should take their dose of PIQRAY at approximately the same time each day.
Swallow PIQRAY tablets whole (tablets should not be chewed, crushed or split prior to swallowing). No tablet should be ingested if it is broken, cracked, or otherwise not intact.
If a dose of PIQRAY is missed, it can be taken with food within 9 hours after the time it is usually taken. After more than 9 hours, skip the dose for that day. The next day, take PIQRAY at the usual time.
If the patient vomits after taking the dose, advise the patient not to take an additional dose on that day, and to resume the dosing schedule the next day at the usual time.
When given with PIQRAY, the recommended dose of fulvestrant is 500 mg administered on Days 1, 15, and 29, and once monthly thereafter. Refer to the Full Prescribing Information for fulvestrant.
2.3 Dose Modifications For Adverse Reactions
The recommended dose modifications for adverse reactions (ARs) are listed in Table 1.
| 1Only one dose reduction is permitted for pancreatitis. 2If further dose reduction below 200 mg once daily is required, discontinue PIQRAY. | ||
| PIQRAY Dose Level | Dose and Schedule | Number and Strength of Tablets |
| Starting dose | 300 mg once daily | Two 150 mg tablets |
| First-dose reduction | 250 mg once daily | One 200 mg tablet and one 50 mg tablet |
| Second-dose reduction | 200 mg once daily2 | One 200 mg tablet |
Tables 2, 3, 4, and 5 summarize recommendations for dose interruption, reduction, or discontinuation of PIQRAY in the management of specific ARs.
Hyperglycemia
| 1FPG/Blood Glucose/Grade levels reflect hyperglycemia grading according to CTCAE Version 4.03 (CTCAE=Common Terminology Criteria for Adverse Events) 2Initiate applicable anti-diabetic medications, including metformin and insulin sensitizers (such as thiazolidinediones or dipeptidyl peptidase-4 inhibitors), and review respective prescribing information for dosing and dose titration recommendations, including local diabetic treatment guidelines. Metformin was recommended in the SOLAR-1 trial with the following guidance: Initiate metformin 500 mg once daily. Based on tolerability, metformin dose may be increased to 500 mg twice daily, followed by 500 mg with breakfast, and 1000 mg with dinner, followed by further increase to 1000 mg twice daily if needed [see Warnings and Precautions (5.3)]. 3As recommended in the SOLAR-1 trial, insulin may be used for 1-2 days until hyperglycemia resolves. However, this may not be necessary in the majority of PIQRAY-induced hyperglycemia, given the short half-life of PIQRAY and the expectation of glucose levels normalizing after interruption of PIQRAY. | |
| Fasting Plasma Glucose (FPG)/Blood Glucose Values1 | Recommendation |
| Grade 1 FPG > ULN-160 mg/dL or > ULN-8.9 mmol/L | No PIQRAY dose adjustment required. Initiate or intensify anti-diabetic treatment2. |
| Grade 2 FPG > 160-250 mg/dL or > 8.9-13.9 mmol/L | No PIQRAY dose adjustment required. Initiate or further intensify anti-diabetic treatment2. If FPG does not decrease to ≤ 160 mg/dL or 8.9 mmol/L within 21 days under appropriate anti-diabetic treatment, reduce PIQRAY dose by 1 dose level and follow FPG value specific recommendations. |
| Grade 3 > 250-500 mg/dL or > 13.9-27.8 mmol/L | Interrupt PIQRAY. Initiate or intensify oral anti-diabetic treatment2 and consider additional anti-diabetic medications3 for 1-2 days until hyperglycemia improves. Administer intravenous hydration and consider appropriate treatment (e.g., intervention for electrolyte/ketoacidosis/hyperosmolar disturbances). If FPG decreases to ≤ 160 mg/dL or 8.9 mmol/L within 3 to 5 days under appropriate anti-diabetic treatment, resume PIQRAY at 1 lower dose level. If FPG does not decrease to ≤ 160 mg/dL or 8.9 mmol/L within 3 to 5 days under appropriate anti-diabetic treatment, consultation with a physician with expertise in the treatment of hyperglycemia is recommended. If FPG does not decrease to ≤ 160 mg/dL or 8.9 mmol/L within 21 days following appropriate anti-diabetic treatment2, permanently discontinue PIQRAY treatment. |
| Grade 4 > 500 mg/dL or ≥ 27.8 mmol/L | Interrupt PIQRAY. Initiate or intensify appropriate anti-diabetic treatment2 (administer intravenous hydration and consider appropriate treatment (e.g., intervention for electrolyte/ketoacidosis/hyperosmolar disturbances)), re-check FPG within 24 hours and as clinically indicated. If FPG decreases to ≤ 500 mg/dL or 27.8 mmol/L, follow FPG value specific recommendations for Grade 3. If FPG is confirmed at > 500 mg/dL or 27.8 mmol/L, permanently discontinue PIQRAY treatment. |
Rash
| 1Grading according to CTCAE Version 5.0 2For all grades of rash, consider consultation with a dermatologist. 3Antihistamines administered prior to rash onset may decrease incidence and severity of rash based on the SOLAR-1 trial. | |
| Grade1,2 | Recommendation3 |
| Grade 1 (< 10% body surface area (BSA) with active skin toxicity) | No PIQRAY dose adjustment required. Initiate topical corticosteroid treatment. Consider adding oral antihistamine to manage symptoms. |
| Grade 2 (10-30% BSA with active skin toxicity) | No PIQRAY dose adjustment required. Initiate or intensify topical corticosteroid and oral antihistamine treatment. Consider low dose systemic corticosteroid treatment. |
| Grade 3 (e.g., severe rash not responsive to medical management) (> 30% BSA with active skin toxicity) | Interrupt PIQRAY. Initiate or intensify topical/systemic corticosteroid and oral antihistamine treatment. Once improved to ≤ Grade 1, resume PIQRAY at the same dose level for first occurrence of rash, or at next lower dose level in case of second occurrence. |
| Grade 4 (e.g., severe bullous, blistering or exfoliating skin conditions) (any % BSA associated with extensive superinfection, with IV antibiotics indicated; life-threatening consequences) | Permanently discontinue PIQRAY. |
Diarrhea
| 1Grading according to CTCAE Version 5.0. | |
| Grade1 | Recommendation |
| Grade 1 | No PIQRAY dose adjustment is required. Initiate appropriate medical therapy and monitor as clinically indicated. |
| Grade 2 | Initiate or intensify appropriate medical therapy and monitor as clinically indicated. Interrupt PIQRAY dose until recovery to Grade ≤ 1, then resume PIQRAY at same dose level. |
| Grade 3 and 4 | Initiate or intensify appropriate medical therapy and monitor as clinically indicated. Interrupt PIQRAY dose until recovery to Grade ≤ 1, then resume PIQRAY at the next lower dose level. |
Other Toxicities
| 1Grading according to CTCAE Version 5.0 2For Grade 2 and 3 pancreatitis, interrupt PIQRAY dose until recovery to Grade < 2 and resume at next lower dose level. Only one dose reduction is permitted. If toxicity reoccurs, permanently discontinue PIQRAY treatment. 3For Grade 2 total bilirubin elevation, interrupt PIQRAY dose until recovery to Grade ≤ 1 and resume at the same dose if resolved in ≤ 14 days or resume at the next lower dose level if resolved in > 14 days. | |
| Grade1 | Recommendation |
| Grade 1 or 2 | No PIQRAY dose adjustment is required. Initiate appropriate medical therapy and monitor as clinically indicated2,3. |
| Grade 3 | Interrupt PIQRAY dose until recovery to Grade ≤ 1, then resume PIQRAY at the next lower dose level. |
| Grade 4 | Permanently discontinue PIQRAY. |
Refer to the Full Prescribing Information of fulvestrant for dose modification guidelines in the event of toxicity and for other relevant safety information.
3 Dosage Forms And Strengths
Tablets: 50 mg, 150 mg, and 200 mg alpelisib
50 mg: Light pink, unscored, round and curved with beveled edges film-coated tablet, imprinted with “L7” on one side and “NVR” on the other side.
150 mg: Pale red, unscored, ovaloid and curved with beveled edges film-coated tablet, imprinted with “UL7” on one side and “NVR” on the other side.
200 mg: Light red, unscored, ovaloid and curved with beveled edges film-coated tablet, imprinted with “YL7” on one side and “NVR” on the other side.
4 Contraindications
PIQRAY is contraindicated in patients with severe hypersensitivity to it or any of its components [see Warnings and Precautions (5.1)].
5.1 Severe Hypersensitivity
Severe hypersensitivity reactions, including anaphylaxis and anaphylactic shock, were reported in patients treated with PIQRAY. Severe hypersensitivity reactions were manifested by symptoms including, but not limited to, dyspnea, flushing, rash, fever, or tachycardia.
The incidence of Grade 3 and 4 hypersensitivity reactions was 0.7% [see Adverse Reactions (6)].
Advise patients of the signs and symptoms of severe hypersensitivity reactions. Permanently discontinue PIQRAY in the event of severe hypersensitivity.
5.2 Severe Cutaneous Reactions
Severe cutaneous reactions, including Stevens-Johnson Syndrome (SJS) and Erythema Multiforme (EM) were reported in patients treated with PIQRAY [see Adverse Reactions (6)].
SJS and EM were reported in 0.4% and 1.1% of patients, respectively. Do not initiate PIQRAY treatment in patients with a history of SJS, EM, or Toxic Epidermal Necrolysis (TEN).
If signs or symptoms of severe cutaneous reactions occur, interrupt PIQRAY until the etiology of the reaction has been determined. Consultation with a dermatologist is recommended.
If SJS, TEN, or EM is confirmed, permanently discontinue PIQRAY. Do not reintroduce PIQRAY in patients who have experienced previous severe cutaneous reactions during PIQRAY treatment.
If SJS, TEN, or EM is not confirmed, PIQRAY may require dose modifications, topical corticosteroids, or oral antihistamine treatment as described in Table 3 [see Dosage and Administration (2.3)].
Advise patients of the signs and symptoms of severe cutaneous reactions (e.g., a prodrome of fever, flu-like symptoms, mucosal lesions or progressive skin rash).
5.3 Hyperglycemia
Severe hyperglycemia, including ketoacidosis, has been reported in patients treated with PIQRAY. Hyperglycemia was reported in 65% of patients treated with PIQRAY. Grade 3 (FPG > 250-500 mg/dL) and Grade 4 (FPG > 500 mg/dL) hyperglycemia was reported in 33% and 3.9% of patients, respectively. Ketoacidosis was reported in 0.7% of patients (n = 2) treated with PIQRAY.
Among the patients who experienced Grade ≥ 2 (FPG 160-250 mg/dL) hyperglycemia, the median time to first occurrence of hyperglycemia was 15 days (range: 5 to 517 days).
In the 187 patients with hyperglycemia, 87% (163/187) were managed with anti-diabetic medication, and 76% (142/187) reported use of metformin as single agent or in combination with other anti-diabetic medication [i.e., insulin, dipeptidyl peptidase-4 (DPP-4) inhibitors, and sulfonylureas]. In patients with Grade ≥ 2 hyperglycemia with at least 1 grade improvement (n = 153), median time to improvement from the first event was 8 days (range: 2 to 65 days).
In all patients with elevated FPG who continued fulvestrant treatment after discontinuing PIQRAY (n = 54), 96% (n = 52) of patients had FPG levels that returned to baseline.
Before initiating treatment with PIQRAY, test FPG, HbA1c, and optimize blood glucose. After initiating treatment with PIQRAY, monitor blood glucose and/or FPG at least once every week for the first 2 weeks, then at least once every 4 weeks, and as clinically indicated. Monitor HbA1c every 3 months and as clinically indicated.
If a patient experiences hyperglycemia after initiating treatment with PIQRAY, monitor blood glucose and/or FPG as clinically indicated, and at least twice weekly until blood glucose or FPG decreases to normal levels. During treatment with anti-diabetic medication, continue monitoring blood glucose or FPG at least once a week for 8 weeks, followed by once every 2 weeks and as clinically indicated. Consider consultation with a healthcare practitioner with expertise in the treatment of hyperglycemia and counsel patients on lifestyle changes.
The safety of PIQRAY in patients with Type 1 and uncontrolled Type 2 diabetes has not been established as these patients were excluded from the SOLAR-1 trial. Patients with a medical history of Type 2 diabetes were included. Patients with a history of diabetes mellitus may require intensified diabetic treatment. Closely monitor patients with diabetes.
Based on the severity of the hyperglycemia, PIQRAY may require dose interruption, reduction, or discontinuation as described in Table 2 [see Dosage and Administration (2.3)].
Advise patients of the signs and symptoms of hyperglycemia (e.g., excessive thirst, urinating more often than usual or higher amount of urine than usual, or increased appetite with weight loss).
5.4 Pneumonitis
Severe pneumonitis, including acute interstitial pneumonitis and interstitial lung disease, has been reported in patients treated with PIQRAY.
Pneumonitis was reported in 1.8% of patients treated with PIQRAY.
In patients who have new or worsening respiratory symptoms or are suspected to have developed pneumonitis, interrupt PIQRAY immediately and evaluate the patient for pneumonitis. Consider a diagnosis of non-infectious pneumonitis in patients presenting with non-specific respiratory signs and symptoms such as hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams and in whom infectious, neoplastic, and other causes have been excluded by means of appropriate investigations.
Permanently discontinue PIQRAY in all patients with confirmed pneumonitis.
Advise patients to immediately report new or worsening respiratory symptoms.
5.5 Diarrhea
Severe diarrhea, including dehydration and acute kidney injury, occurred in patients treated with PIQRAY. Most patients (58%) experienced diarrhea during treatment with PIQRAY. Grade 3 diarrhea occurred in 7% (n = 19) of patients. Among patients with Grade 2 or 3 diarrhea (n = 71), the median time to onset was 46 days (range: 1 to 442 days).
Dose reductions of PIQRAY were required in 6% of patients and 2.8% of patients permanently discontinued PIQRAY due to diarrhea. In the 164 patients that experienced diarrhea, anti-diarrheal medications (e.g., loperamide) were required to manage symptoms in 63% (104/164) of these patients.
Based on the severity of the diarrhea, PIQRAY may require dose interruption, reduction, or discontinuation as described in Table 4 [see Dosage and Administration (2.3)].
Advise patients to start antidiarrheal treatment, increase oral fluids, and notify their healthcare provider if diarrhea occurs while taking PIQRAY.
5.6 Embryo-Fetal Toxicity
Based on findings in animals and its mechanism of action, PIQRAY can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, oral administration of alpelisib to pregnant rats and rabbits during organogenesis caused adverse developmental outcomes including embryo-fetal mortality (post-implantation loss), reduced fetal weights, and increased incidences of fetal malformations at maternal exposures based on area under the curve (AUC) that were ≥ 0.8 times the exposure in humans at the recommended dose of 300 mg/day. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with PIQRAY and for 1 week after the last dose. Advise male patients with female partners of reproductive potential to use condoms and effective contraception during treatment with PIQRAY and for 1 week after the last dose [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
Refer to the Full Prescribing Information of fulvestrant for pregnancy and contraception information.
6 Adverse Reactions
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Severe Hypersensitivity [see Warnings and Precautions (5.1)]
- Severe Cutaneous Reactions [see Warnings and Precautions (5.2)]
- Hyperglycemia [see Warnings and Precautions (5.3)]
- Pneumonitis [see Warnings and Precautions (5.4)]
- Diarrhea [see Warnings and Precautions (5.5)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of PIQRAY was evaluated in a randomized, double-blind, placebo-controlled trial (SOLAR-1) in 571 patients with HR-positive, HER2-negative, advanced or metastatic breast cancer enrolled into two cohorts, with or without a PIK3CA mutation [see Clinical Studies (14)].
Patients received either PIQRAY 300 mg plus fulvestrant (n = 284) or placebo plus fulvestrant (n = 287). Fulvestrant 500 mg was administered intramuscularly on Cycle 1, Day 1 and 15 and then at Day 1 of each 28-day cycle during treatment phase.
Two patients (0.7%) died while on treatment with PIQRAY plus fulvestrant due to causes other than the underlying malignancy. Causes of death included one cardio-respiratory arrest and one second primary malignancy. Neither was suspected to be related to study treatment.
Serious adverse reactions occurred in 35% of patients receiving PIQRAY plus fulvestrant. Serious adverse reactions in > 2% of patients receiving PIQRAY plus fulvestrant included hyperglycemia (10%), rash (3.5%), diarrhea (2.8%), acute kidney injury (2.5%), abdominal pain (2.1%), and anemia (2.1%).
Osteonecrosis of the jaw (ONJ) was reported in 4.2% of patients (12/284) in the PIQRAY plus fulvestrant arm compared to 1.4% of patients (4/287) in the placebo arm. All patients experiencing ONJ had prior or concomitant bisphosphonates or RANK-ligand inhibitor administration.
Among patients receiving PIQRAY plus fulvestrant, 4.6% permanently discontinued both PIQRAY and fulvestrant and 21% permanently discontinued PIQRAY alone, due to ARs. The most frequent ARs leading to treatment discontinuation of PIQRAY in > 2% patients receiving PIQRAY plus fulvestrant were hyperglycemia (6%), rash (4.2%), diarrhea (2.8%), and fatigue (2.5%).
Dose reductions due to ARs occurred in 55% of patients receiving PIQRAY plus fulvestrant. The most frequent ARs leading to dose reduction in > 2% patients receiving PIQRAY plus fulvestrant were hyperglycemia (29%), rash (9%), diarrhea (6%), stomatitis (3.5%) and mucosal inflammation (2.1%).
The most common adverse reactions including laboratory abnormalities (all grades, incidence ≥ 20%) were glucose increased, creatinine increased, diarrhea, rash, lymphocyte count decreased, GGT increased, nausea, ALT increased, fatigue, hemoglobin decreased, lipase increased, decreased appetite, stomatitis, vomiting, weight decreased, calcium decreased, glucose decreased, aPTT prolonged, and alopecia.
Adverse reactions and laboratory abnormalities are listed in Table 6 and Table 7, respectively.
| Grading according to CTCAE Version 4.03 1Stomatitis: including stomatitis, aphthous ulcer and mouth ulceration 2Abdominal pain: abdominal pain, abdominal pain upper, abdominal pain lower 3Fatigue: including fatigue, asthenia 4Mucosal dryness: including dry mouth, mucosal dryness, vulvovaginal dryness 5Urinary tract infection: including UTI and single case of urosepsis 6Dysgeusia: including dysgeusia, ageusia, hypogeusia 7Rash: including rash, rash maculo-papular, rash macular, rash generalized, rash papular, rash pruritic 8Dry skin: including dry skin, skin fissures, xerosis, xeroderma *No Grade 4 adverse reactions were reported. | ||||
| PIQRAY plus fulvestrant N = 284 | Placebo plus fulvestrant N = 287 | |||
| Adverse reactions | All Grades | Grade 3-4 | All Grades | Grade 3-4 |
| % | % | % | % | |
| Gastrointestinal disorders | ||||
| Diarrhea | 58 | 7* | 16 | 0.3* |
| Nausea | 45 | 2.5* | 22 | 0.3* |
| Stomatitis1 | 30 | 2.5* | 6 | 0* |
| Vomiting | 27 | 0.7* | 10 | 0.3* |
| Abdominal pain2 | 17 | 1.4* | 11 | 1* |
| Dyspepsia | 11 | 0* | 6 | 0* |
| General disorders and administration site conditions | ||||
| Fatigue3 | 42 | 5* | 29 | 1* |
| Mucosal inflammation | 19 | 2.1* | 1 | 0* |
| Edema peripheral | 15 | 0* | 5 | 0.3* |
| Pyrexia | 14 | 0.7 | 4.9 | 0.3* |
| Mucosal dryness4 | 12 | 0.4* | 4.2 | 0* |
| Infections and infestations | ||||
| Urinary tract infection5 | 10 | 0.7* | 5 | 1* |
| Investigations | ||||
| Weight decreased | 27 | 3.9* | 2.1 | 0* |
| Metabolism and nutrition disorders | ||||
| Decreased appetite | 36 | 0.7* | 10 | 0.3* |
| Nervous system disorders | ||||
| Dysgeusia6 | 18 | 0.4* | 3.5 | 0* |
| Headache | 18 | 0.7* | 13 | 0* |
| Skin and subcutaneous tissue disorders | ||||
| Rash7 | 52 | 20* | 7 | 0.3* |
| Alopecia | 20 | 0* | 2.4 | 0* |
| Pruritus | 18 | 0.7* | 6 | 0* |
| Dry skin8 | 18 | 0.4* | 3.8 | 0* |
Among the patients with Grade 2 or 3 rash, the median time to first onset of Grade 2 or 3 rash was 12 days. A subgroup of 86 patients received prophylaxis, including anti-histamines, prior to onset of rash. In these patients, rash was reported less frequently than in the overall population, for all grades rash (27% vs 54%), Grade 3 rash (12% vs 20%) and rash leading to permanent discontinuation of PIQRAY (3.5% vs 4.2%). Of the 153 patients who experienced rash, 141 had resolution of the rash.
| 1Glucose increase is an expected laboratory abnormality of PI3K inhibition. *No Grade 4 laboratory abnormalities were reported. | ||||||
| PIQRAY plus fulvestrant N = 284 | Placebo plus fulvestrant N = 287 | |||||
| Laboratory Abnormality | All Grades | Grade 3-4 | All Grades | Grade 3-4 | ||
| % | % | % | % | |||
| Hematological parameters | ||||||
| Lymphocyte count decreased | 52 | 8 | 40 | 4.5* | ||
| Hemoglobin decreased | 42 | 4.2* | 29 | 1* | ||
| Activated Partial Thromboplastin Time (aPTT) prolonged | 21 | 0.7* | 16 | 0.3* | ||
| Platelet count decreased | 14 | 1.1 | 6 | 0* | ||
| Biochemical parameters | ||||||
| Glucose increased1 | 79 | 39 | 34 | 1 | ||
| Creatinine increased | 67 | 2.8* | 25 | 0.7* | ||
| Gamma Glutamyl Transferase (GGT) increased | 52 | 11 | 44 | 10 | ||
| Alanine Aminotransferase (ALT) increased | 44 | 3.5 | 34 | 2.4* | ||
| Lipase increased | 42 | 7 | 25 | 6 | ||
| Calcium (corrected) decreased | 27 | 2.1 | 20 | 1.4 | ||
| Glucose decreased | 26 | 0.4 | 14 | 0* | ||
| Potassium decreased | 14 | 6 | 2.8 | 0.7* | ||
| Albumin decreased | 14 | 0* | 8 | 0* | ||
| Magnesium decreased | 11 | 0.4* | 4.2 | 0* | ||
7.1 Effect Of Other Drugs On Piqray
CYP3A4 Inducer
Coadministration of PIQRAY with a strong CYP3A4 inducer may decrease alpelisib concentration [see Clinical Pharmacology (12.3)], which may decrease alpelisib activity. Avoid coadministration of PIQRAY with strong CYP3A4 inducers.
BCRP Inhibitors
Coadministration of PIQRAY with a BCRP inhibitor may increase alpelisib concentration [see Clinical Pharmacology (12.3)], which may increase the risk of toxicities. Avoid the use of BCRP inhibitors in patients treated with PIQRAY. If unable to use alternative drugs, when PIQRAY is used in combination with BCRP inhibitors, closely monitor for increased adverse reactions.
7.2 Effect Of Piqray On Other Drugs
CYP2C9 Substrates
Coadministration of PIQRAY with CYP2C9 substrates (e.g., warfarin) may reduce plasma concentration of these drugs [see Clinical Pharmacology (12.3)]. Closely monitor when PIQRAY is used in combination with CYP2C9 substrates where decreases in the plasma concentration of CYP2C9 substrates may reduce activity of these drugs.
8.1 Pregnancy
Risk Summary
PIQRAY is used in combination with fulvestrant. Refer to the Full Prescribing Information of fulvestrant for pregnancy information.
Based on animal data and mechanism of action, PIQRAY can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, oral administration of alpelisib to pregnant rats and rabbits during organogenesis caused adverse developmental outcomes including embryo-fetal mortality (post-implantation loss), reduced fetal weights, and increased incidences of fetal malformations at maternal exposures ≥ 0.8 times the exposure in humans based on AUC at the recommended dose of 300 mg/day (see Data). Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. However, the estimated background risk of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies in the U.S. general population.
Data
Animal Data
In embryo-fetal development studies in rats and rabbits, pregnant animals received oral doses of alpelisib up to 30 mg/kg/day during the period of organogenesis.
In rats, oral administration of alpelisib resulted in maternal toxicity (body weight loss, low food consumption) and no viable fetuses (post-implantation loss) at 30 mg/kg/day (approximately 3 times the exposure in humans at the recommended dose of 300 mg/day based on AUC). At a dose of 10 mg/kg/day (approximately 0.8 times the exposure in humans at the recommended dose of 300 mg/day based on AUC), toxicities included reduced fetal weight and increased incidences of skeletal malformations (bent scapula and thickened or bent long bones) and fetal variations (enlarged brain ventricle, decreased bone ossification).
In a pilot embryo-fetal development study in rabbits, a dose of 30 mg/kg/day resulted in no viable fetuses (post-implantation loss). Doses ≥ 15 mg/kg/day resulted in increased embryo-fetal deaths, reduced fetal weights, and malformations, mostly related to the tail and head. At 15 mg/kg/day in rabbits, the maternal exposure was approximately 5 times the exposure achieved at the recommended human dose of 300 mg/day based on AUC.
8.2 Lactation
PIQRAY is used in combination with fulvestrant. Refer to the Full Prescribing Information of fulvestrant for lactation information.
There is no data on the presence of alpelisib in human milk, its effects on milk production, or the breastfed child. Because of the potential for serious adverse reactions in the breastfed child, advise lactating women to not breastfeed during treatment with PIQRAY and for 1 week after the last dose.
8.3 Females And Males Of Reproductive Potential
PIQRAY is used in combination with fulvestrant. Refer to the Full Prescribing Information of fulvestrant for contraception and infertility information.
Pregnancy Testing
Verify the pregnancy status in females of reproductive potential prior to initiating PIQRAY.
Contraception
Females
PIQRAY can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with PIQRAY and for 1 week after the last dose.
Males
Advise male patients with female partners of reproductive potential to use condoms and effective contraception during treatment with PIQRAY and for 1 week after the last dose.
Infertility
Based on findings from animal studies, PIQRAY may impair fertility in males and females of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and efficacy of PIQRAY in pediatric patients have not been established.
8.5 Geriatric Use
Of 284 patients who received PIQRAY in the SOLAR-1 trial, 117 patients were ≥ 65 years of age and 34 patients were ≥ 75 years of age. In patients treated with PIQRAY plus fulvestrant, there was a higher incidence of Grade 3-4 hyperglycemia in patients ≥ 65 years of age (44%) compared to patients < 65 years of age (32%). No overall differences in effectiveness of PIQRAY were observed between patients ≥ 65 years of age compared to younger patients. There are an insufficient number of patients ≥ 75 years of age to assess whether there are differences in safety or effectiveness.
8.6 Renal Impairment
The effect of severe renal impairment (CLcr < 30 mL/min) on alpelisib pharmacokinetics is unknown [see Clinical Pharmacology (12.3)].
No dose adjustment is recommended for patients with mild to moderate renal impairment (CLcr 30 to < 90 mL/min).
10 Overdosage
There is limited experience of overdose with PIQRAY in clinical trials. In the clinical studies, PIQRAY was administered at doses up to 450 mg once daily.
In cases where accidental overdosage of PIQRAY was reported in the clinical studies, the adverse reactions associated with the overdose were consistent with the known safety profile of PIQRAY and included hyperglycemia, nausea, asthenia, and rash.
Initiate general symptomatic and supportive measures in all cases of overdosage where necessary. There is no known antidote for PIQRAY.
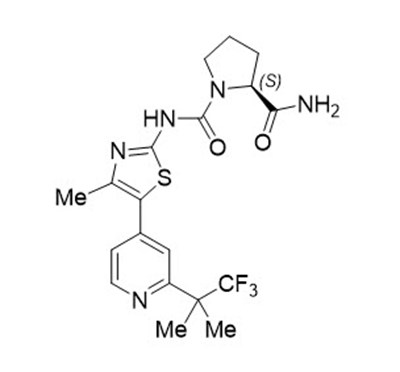
11 Description
PIQRAY (alpelisib) is a kinase inhibitor. The chemical name of alpelisib is (2S)-N1-[4-Methyl-5-[2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridinyl]-2-thiazolyl]-1,2-pyrrolidinedicarboxamide. Alpelisib is a white to almost white powder. The molecular formula for alpelisib is C19H22F3N5O2S and the relative molecular mass is 441.47 g/mol. The chemical structure of alpelisib is shown below:
PIQRAY film-coated tablets are supplied for oral administration with three strengths that contain 50 mg, 150 mg and 200 mg of alpelisib. The tablets also contain hypromellose, magnesium stearate, mannitol, microcrystalline cellulose, and sodium starch glycolate. The film-coating contains hypromellose, iron oxide black, iron oxide red, macrogol/polyethylene glycol (PEG) 4000, talc, and titanium dioxide.
12.1 Mechanism Of Action
Alpelisib is an inhibitor of phosphatidylinositol-3-kinase (PI3K) with inhibitory activity predominantly against PI3Kα. Gain-of-function mutations in the gene encoding the catalytic α-subunit of PI3K (PIK3CA) lead to activation of PI3Kα and Akt-signaling, cellular transformation and the generation of tumors in in vitro and in vivo models.
In breast cancer cell lines, alpelisib inhibited the phosphorylation of PI3K downstream targets, including Akt and showed activity in cell lines harboring a PIK3CA mutation. In vivo, alpelisib inhibited the PI3K/Akt signaling pathway and reduced tumor growth in xenograft models, including models of breast cancer.
PI3K inhibition by alpelisib treatment has been shown to induce an increase in estrogen receptor (ER) transcription in breast cancer cells. The combination of alpelisib and fulvestrant demonstrated increased anti-tumor activity compared to either treatment alone in xenograft models derived from ER-positive, PIK3CA mutated breast cancer cell lines.
12.2 Pharmacodynamics
Cardiac Electrophysiology
Serial ECGs were collected following a single dose and at steady-state to evaluate the effect of alpelisib on the QTcF interval in patients with advanced cancer. An analysis of clinical ECG data demonstrates the absence of a large effect (i.e., > 20 ms) on QTcF prolongation at the recommended 300 mg dose with or without fulvestrant.
12.3 Pharmacokinetics
The pharmacokinetics of alpelisib has been studied in healthy subjects and adult patients with solid tumors. Steady-state alpelisib maximum plasma concentration (Cmax) and AUC increased proportionally over the dose range of 30 mg to 450 mg (0.1 to 1.5 times the approved recommended dosage) under fed conditions. The mean accumulation of alpelisib is 1.3 to 1.5 and steady-state plasma concentrations are reached within 3 days following daily dosage. In adult patients who received PIQRAY 300 mg once daily in the SOLAR-1 trial, population approach derived mean steady-state alpelisib [coefficient of variation (CV%)] for Cmax was 2480 (23%) ng/mL and AUC0-24hr was 33224 (21%) ng*h/mL.
Absorption
The median time to reach peak plasma concentration (Tmax) ranged between 2.0 to 4.0 hours.
Effect of food
A high-fat high-calorie meal (985 calories with 58.1 g of fat) increased alpelisib AUC by 73% and Cmax by 84%, and a low-fat low-calorie meal (334 calories with 8.7 g of fat) increased alpelisib AUC by 77% and Cmax by 145% following a single dose of PIQRAY. No clinically significant differences in alpelisib AUC were observed between low-fat low-calorie and high-fat high-calorie meals.
Distribution
The mean (% CV) apparent volume of distribution of alpelisib at steady-state is predicted to be 114 L (46%). Protein binding of alpelisib is 89% and is independent of concentration.
Elimination
The half-life of alpelisib is predicted to be 8 to 9 hours. The mean (% CV) clearance of alpelisib is predicted to be 9.2 L/hr (21%) under fed conditions.
Metabolism
Alpelisib is primarily metabolized by chemical and enzymatic hydrolysis to form its metabolite BZG791 and to a lesser extent by CYP3A4, in vitro.
Excretion
Following a single oral dose of 400 mg radiolabeled alpelisib under fasted condition, 81% of the administered dose was recovered in feces (36% unchanged, 32% BZG791) and 14% (2% unchanged, 7.1% BZG791) in urine. CYP3A4-mediated metabolites (12%) and glucuronides amounted to approximately 15% of the dose.
Specific Populations
No clinically significant differences in the pharmacokinetics of alpelisib were predicted based on age (21 to 87 years), sex, race/ethnicity (Japanese or Caucasian), body weight (37 to 181 kg), mild to moderate renal impairment (CLcr 30 to < 90 mL/min based on the Cockcroft-Gault formula), or mild to severe hepatic impairment (Child-Pugh Class A, B, and C). The effect of severe renal impairment (CLcr < 30 mL/min) on the pharmacokinetics of alpelisib is unknown.
Drug Interaction Studies
Clinical Studies
Acid Reducing Agents: PIQRAY can be coadministered with acid reducing agents, since PIQRAY should be taken with food. Food exhibited a more pronounced effect on the solubility of alpelisib than the effect of gastric pH value.
Coadministration of the H2 receptor antagonist ranitidine in combination with a single 300 mg oral dose of alpelisib decreased the absorption and overall exposure of alpelisib. In the presence of a low-fat low-calorie meal, AUC was decreased on average by 21% and Cmax by 36% with ranitidine. Under the fasted state, AUC was decreased on average by 30% and Cmax by 51% with ranitidine.
CYP3A4 Substrates: No clinically significant differences in pharmacokinetics of everolimus (a substrate of CYP3A4 and P-gp) were observed when coadministered with alpelisib.
In Vitro Studies
Effect of Alpelisib on CYP Enzymes: Alpelisib inhibits CYP3A4 in a time-dependent manner and induces CYP2B6, CYP2C9 and CYP3A4.
Effect of Transporter on Alpelisib: Alpelisib is a substrate of BCRP.
Effect of Alpelisib on Transporters: Alpelisib is an inhibitor of P-gp. Alpelisib has a low potential to inhibit BCRP, MRP2, BSEP, OATP1B1, OATP1B3, OCT1, OAT1, OAT3, OCT2, MATE1, and MATE2K at clinically relevant concentrations.
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenicity studies have not been conducted with alpelisib.
Alpelisib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay, or aneugenic or clastogenic in human cell micronucleus and chromosome aberration tests in vitro. Alpelisib was not genotoxic in an in vivo rat micronucleus test.
Fertility studies in animals have not been conducted. In repeated-dose toxicity studies up to 13 weeks duration, adverse effects were observed in reproductive organs including vaginal atrophy and estrous cycle variations in rats at doses ≥ 6 mg/kg/day (approximately 0.6 times the exposure in humans at the recommended dose of 300 mg/day based on AUC), and prostate atrophy in dogs at doses ≥ 15 mg/kg/day (approximately 2.6 times the exposure in humans at the recommended dose of 300 mg/day based on AUC).
14 Clinical Studies
SOLAR-1 (NCT02437318) was a randomized, double-blind, placebo-controlled trial of PIQRAY plus fulvestrant versus placebo plus fulvestrant in 572 patients with HR-positive, HER2-negative, advanced or metastatic breast cancer whose disease had progressed or recurred on or after an aromatase inhibitor-based treatment (with or without CDK4/6 combination). Patients were excluded if they had inflammatory breast cancer, diabetes mellitus Type 1 or uncontrolled Type 2, or pneumonitis. Randomization was stratified by presence of lung and/or liver metastasis and previous treatment with CDK4/6 inhibitor(s). Overall, 60% of enrolled patients had tumors with one or more PIK3CA mutations in tissue, 50% had liver/lung metastases, and 6% had previously been treated with a CDK4/6 inhibitor.
There were 341 patients enrolled by tumor tissue in the cohort with a PIK3CA mutation and 231 enrolled in the cohort without a PIK3CA mutation. Of the 341 patients in the cohort with a PIK3CA mutation, 336 (99%) patients had one or more PIK3CA mutations confirmed in tumor tissue using the FDA-approved therascreen® PIK3CA RGQ PCR Kit. Out of the 336 patients with PIK3CA mutations confirmed in tumor tissue, 19 patients had no plasma specimen available for testing with the FDA-approved therascreen® PIK3CA RGQ PCR Kit. Of the remaining 317 patients with PIK3CA mutations confirmed in tumor tissue, 177 patients (56%) had PIK3CA mutations identified in plasma specimen, and 140 patients (44%) did not have PIK3CA mutations identified in plasma specimen.
Patients received either PIQRAY (300 mg) or placebo orally once daily on a continuous basis, plus fulvestrant (500 mg) administered intramuscularly on Cycle 1, Days 1 and 15, and then on Day 1 of every 28-day cycle. Patients received treatment until radiographic disease progression or unacceptable toxicity. Tumor assessments were performed every 8 weeks for the first 18 months and every 12 weeks thereafter.
The median age of patients was 63 years (range 25 to 92). Most patients were women (99.8%) and most patients were White (66%), followed by Asian (22%), Other/Unknown (10%), Black or African American (1.4%), and American Indian or Alaskan Native (0.9%). Baseline ECOG performance status was 0 (68%) or 1 (32%).
Patient demographics for those with PIK3CA-mutated tumors were generally representative of the broader study population. The median duration of exposure to PIQRAY plus fulvestrant was 8.2 months with 59% of patients exposed for > 6 months.
The majority of patients (98%) received prior hormonal therapy as the last treatment (48% metastatic setting, 52% adjuvant setting). Primary endocrine resistance, defined as relapsed within 24 months on adjuvant endocrine therapy or progression within 6 months on endocrine therapy for advanced disease, was observed in 13% of patients and secondary endocrine resistance, defined as relapsed after 24 months on adjuvant endocrine therapy, relapsed within 12 months of the end of adjuvant endocrine therapy, or progression after 6 months on endocrine therapy for advanced disease, was observed in 72% of patients.
The major efficacy outcome was investigator-assessed progression-free survival (PFS) in the cohort with a PIK3CA mutation per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Additional efficacy outcome measures were overall response rate (ORR) and overall survival (OS) in the cohort with a PIK3CA mutation.
Efficacy results for the cohort with a PIK3CA mutation in tumor tissue are presented in Table 8 and Figure 1. PFS results for the cohort with a PIK3CA mutation by investigator assessment were supported by consistent results from a blinded independent review committee (BIRC) assessment. Consistent results were seen in patients with tissue or plasma PIK3CA mutations. At the time of final PFS analysis, 27% (92/341) of patients had died, and overall survival follow-up was immature.
No PFS benefit was observed in patients whose tumors did not have a PIK3CA tissue mutation (HR = 0.85; 95% CI: 0.58, 1.25).
| 1Both log-rank test and Cox proportional hazards model are stratified by prior CDK4/6 inhibitor usage and presence of lung/liver metastases. P-value was compared to prespecified Haybittle-Peto stopping boundary (two-sided p ≤ 0.0398). 2ORR = percentage of patients with confirmed Complete Response or Partial Response with measurable disease at baseline | |||
| PIQRAY plus fulvestrant | Placebo plus fulvestrant | ||
| Progression-free survival | N = 169 | N = 172 | |
| Number of PFS events – n (%) | 103 (61) | 129 (75) | |
| Median PFS months (95% CI) | 11.0 (7.5, 14.5) | 5.7 (3.7, 7.4) | |
| Hazard ratio (95% CI) | 0.65 (0.50, 0.85) | ||
| p-value1 | 0.0013 | ||
| Overall Response Rate | N = 126 | N = 136 | |
| ORR2 (95% CI) | 35.7 (27.4, 44.7) | 16.2 (10.4, 23.5) | |
Figure 1: Progression Free Survival in SOLAR-1 (Per Investigator Assessment of Patients with a PIK3CA Tumor Mutation)
16 How Supplied/Storage And Handling
PIQRAY (alpelisib) 50 mg, 150 mg, and 200 mg film-coated tablets [see Dosage Forms and Strengths (3)].
300 mg daily dose: Each carton contains 2 blister packs. Each blister pack contains a 14-day supply of 28 tablets (28 tablets, 150 mg alpelisib per tablet). NDC 0078-0708-02
250 mg daily dose: Each carton contains 2 blister packs. Each blister pack contains a 14-day supply of 28 tablets (14 tablets, 200 mg alpelisib per tablet and 14 tablets, 50 mg alpelisib per tablet). NDC 0078-0715-02
200 mg daily dose: Each carton contains 1 blister pack. Each blister pack contains a 28-day supply of 28 tablets (28 tablets, 200 mg alpelisib per tablet). NDC 0078-0701-84
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
17 Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Severe Hypersensitivity
Inform patients of the signs and symptoms of hypersensitivity. Advise patients to contact their healthcare provider immediately for signs and symptoms of hypersensitivity [see Warnings and Precautions (5.1)].
Severe Cutaneous Reactions
Inform patients of the signs and symptoms of severe cutaneous reactions. Advise patients to contact their healthcare provider immediately for signs and symptoms of severe cutaneous reactions [see Warnings and Precautions (5.2)].
Hyperglycemia
Advise patients of the possibility of developing hyperglycemia and the need to monitor blood glucose periodically during therapy. Advise patients to contact their healthcare provider immediately for signs and symptoms of hyperglycemia [see Warnings and Precautions (5.3)].
Pneumonitis
Inform patients of the possibility of developing pneumonitis and to immediately contact their healthcare provider if they experience respiratory problems [see Warnings and Precautions (5.4)].
Diarrhea
Advise patients that PIQRAY may cause diarrhea, which may be severe in some cases. Inform patients to start antidiarrheal treatment, increase oral fluids, and notify their healthcare provider if diarrhea occurs while taking PIQRAY [see Warnings and Precautions (5.5)].
Embryo-Fetal Toxicity
- Inform pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.6) and Use in Specific Populations (8.1)].
- Advise females of reproductive potential to use effective contraception during treatment with PIQRAY and for 1 week after the last dose [see Use in Specific Populations (8.3)].
- Advise male patients with female partners of reproductive potential to use condoms and effective contraception during treatment with PIQRAY and for 1 week after the last dose [see Use in Specific Populations (8.3)].
- Refer to the Full Prescribing Information of fulvestrant for pregnancy and contraception information.
- Instruct patients to take PIQRAY at approximately the same time each day and to swallow the tablet(s) whole (tablets should not be chewed, crushed, or split prior to swallowing) [see Dosage and Administration (2.2)].
- Advise patients to take PIQRAY with food [see Drug Interactions (7.4)].
- Instruct patients that if a dose of PIQRAY is missed, it can be taken with food within 9 hours after the time it is usually taken. After more than 9 hours, skip the dose for that day. The next day, take PIQRAY at the usual time. Instruct patients not to take 2 doses to make up for a missed dose.
- Instruct patients that if they vomit after taking the dose of PIQRAY, they should not take an additional dose on that day, and to resume the usual dosing schedule the next day at the usual time [see Dosage and Administration (2.2)].
Lactation
Advise women not to breastfeed during treatment with PIQRAY and for 1 week after the last dose [see Use in Specific Populations (8.2)]. Refer to the Full Prescribing Information of fulvestrant for lactation information.
Infertility
Advise males and females of reproductive potential that PIQRAY may impair fertility [see Use in Specific Populations (8.3)]. Refer to the Full Prescribing Information of fulvestrant for infertility information.
Drug Interactions
Advise patients to avoid the use of strong CYP3A4 inducers in patients treated with PIQRAY. Advise patients to avoid the use of BCRP inhibitors in patients treated with PIQRAY. If unable to use alternative drugs, closely monitor for increased adverse reactions. Advise patients that close monitoring may be required when PIQRAY is coadministered with CYP2C9 substrates where decreases in the plasma concentration of CYP2C9 substrates may reduce activity of these drugs [see Drug Interactions (7.1, 7.2)].
Dosing
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
© Novartis
T2019-57
* Please review the disclaimer below.