Product Images Breo Ellipta
View Photos of Packaging, Labels & Appearance
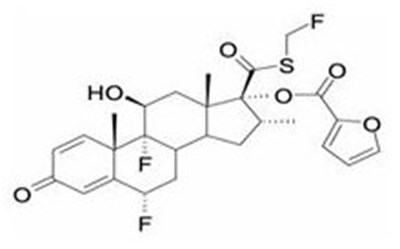
- fluticasone furoate chemical structure - breo ellipta spl graphic 01
- vilanterol trifenatate chemical structure - breo ellipta spl graphic 02
- Figure 1. Impact of Intrinsic Factors on the Pharmacokinetics (PK) of Fluticasone Furoate (FF) and Vilanterol (VI) Following Administration as Fluticasone Furoate/Vilanterol Combination - breo ellipta spl graphic 03
- Figure 2. Impact of Coadministered Drugsa on the Pharmacokinetics (PK) of Fluticasone Furoate (FF) and Vilanterol (VI) Following Administration as Fluticasone Furoate/Vilanterol Combination or Vilanterol Coadministered with a Long-Acting Muscarinic Antagonist - breo ellipta spl graphic 04
- Figure 3. Least Squares (LS) Mean Change from Baseline in Postdose Serial FEV1 (0-24 h) (mL) on Days 1 and 28, Day 1 - breo ellipta spl graphic 05
- Figure 3. Least Squares (LS) Mean Change from Baseline in Postdose Serial FEV1 (0-24 h) (mL) on Days 1 and 28, Day 28 - breo ellipta spl graphic 06
- Figure 4. Raw Mean Change from Baseline in Postdose Serial FEV1 (0-4 h) (mL) on Days 1 and 168, Day 1 - breo ellipta spl graphic 07
- Figure 4. Raw Mean Change from Baseline in Postdose Serial FEV1 (0-4 h) (mL) on Days 1 and 168, Day 168 - breo ellipta spl graphic 08
- Figure 5. Least Squares (LS) Mean Change from Baseline in Postdose Serial FEV1 (0-24 h) (mL) on Days 1 and 28, Day 1 - breo ellipta spl graphic 09
- Figure 5. Least Squares (LS) Mean Change from Baseline in Postdose Serial FEV1 (0-24 h) (mL) on Days 1 and 28, Day 28 - breo ellipta spl graphic 10
- Figure 6: Fluticasone Furoate Dose-Ranging and Dose-Frequency Trials - breo ellipta spl graphic 11
- Figure 7. Least Squares (LS) Mean Change from Baseline in Individual Serial FEV1 (mL) Assessments over 24 Hours at Day 1 (Trial 1) - breo ellipta spl graphic 12
- Parts figure - breo ellipta spl graphic 13
- Figure A - breo ellipta spl graphic 14
- Figure B - breo ellipta spl graphic 15
- Figure C - breo ellipta spl graphic 16
- Figure D - breo ellipta spl graphic 17
- Figure E - breo ellipta spl graphic 18
- Figure F - breo ellipta spl graphic 19
- Figure G - breo ellipta spl graphic 20
- Figure H - breo ellipta spl graphic 21
- Figure I - breo ellipta spl graphic 22
- Figure J - breo ellipta spl graphic 23
- Fig K - breo ellipta spl graphic 24
- Figure K - breo ellipta spl graphic 25
- Breo Ellipta 100mcg-25mcg 30 dose carton - breo ellipta spl graphic 26
- Breo Ellipta 200 mcg-25mcg 30 dose carton - breo ellipta spl graphic 27
Product Label Images
The following 27 images provide visual information about the product associated with Breo Ellipta NDC 0173-0882 by Glaxosmithkline Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1. Impact of Intrinsic Factors on the Pharmacokinetics (PK) of Fluticasone Furoate (FF) and Vilanterol (VI) Following Administration as Fluticasone Furoate/Vilanterol Combination - breo ellipta spl graphic 03

Severe renal and hepatic impairment has been observed in individuals aged over 65 years belonging to the Asian population and of both genders. The text contains a fold change and percentage values. There are recommendations for "no dose adjustment".*
Figure 2. Impact of Coadministered Drugsa on the Pharmacokinetics (PK) of Fluticasone Furoate (FF) and Vilanterol (VI) Following Administration as Fluticasone Furoate/Vilanterol Combination or Vilanterol Coadministered with a Long-Acting Muscarinic Antagonist - breo ellipta spl graphic 04

Figure 3. Least Squares (LS) Mean Change from Baseline in Postdose Serial FEV1 (0-24 h) (mL) on Days 1 and 28, Day 1 - breo ellipta spl graphic 05

This is a statistical table showing changes in LS mean from a baseline measurement for several different treatments. The treatments are listed, along with their dosages. However, without additional context or a key for the columns, it is difficult to interpret the specific values in the table.*
Figure 3. Least Squares (LS) Mean Change from Baseline in Postdose Serial FEV1 (0-24 h) (mL) on Days 1 and 28, Day 28 - breo ellipta spl graphic 06

This is a statistical table indicating the LS mean change from baseline in units of L by time (in thousands) for various treatments involving different doses of Vianterol and Ulanterol, including a placebo.*
Figure 4. Raw Mean Change from Baseline in Postdose Serial FEV1 (0-4 h) (mL) on Days 1 and 168, Day 1 - breo ellipta spl graphic 07

Not available.*
Figure 4. Raw Mean Change from Baseline in Postdose Serial FEV1 (0-4 h) (mL) on Days 1 and 168, Day 168 - breo ellipta spl graphic 08

The text provides a graph displaying mean change from baseline FEVa (Forced Expiratory Volume in One Second) in mL after taking various medications. The medications included are Breo Ellipta 100/25, Breo Ellipta 200/25, Vilanterol 25mcg, and Fluticasone Furoate 200mcg. There is also a placebo represented on the graph. The x-axis represents time since dose in minutes, and the y-axis represents the mL change from baseline FEVa.*
Figure 5. Least Squares (LS) Mean Change from Baseline in Postdose Serial FEV1 (0-24 h) (mL) on Days 1 and 28, Day 1 - breo ellipta spl graphic 09

Figure 5. Least Squares (LS) Mean Change from Baseline in Postdose Serial FEV1 (0-24 h) (mL) on Days 1 and 28, Day 28 - breo ellipta spl graphic 10

Figure 7. Least Squares (LS) Mean Change from Baseline in Individual Serial FEV1 (mL) Assessments over 24 Hours at Day 1 (Trial 1) - breo ellipta spl graphic 12

The given text appears to be a graph depicting the LS mean change from baseline in milliliters at different time intervals since taking the drug BREO ELLIPTA 200/25 with Fluticasone furoate 100mcg. However, the readability of the text is compromised due to errors resulting in missing values and characters. Therefore, an accurate interpretation of the graph cannot be provided.*
Parts figure - breo ellipta spl graphic 13

This is a very short text, only two words, and it describes a product, a "tray lid" that is most likely made out of carton. It is not clear what kind of tray or what size. There is not enough information for a detailed description, but it can be assumed that it is a cover for a container, tray or box made out of carton.*
Figure G - breo ellipta spl graphic 20

This is a warning message informing users not to block the air vent with their fingers.*
Breo Ellipta 100mcg-25mcg 30 dose carton - breo ellipta spl graphic 26

BREO Ellipta is a prescription medicine that contains two active ingredients, fluticasone furoate and vilanterol, and is used for oral inhalation only. It comes in the form of inhalation powder and is contained in a device called ELLIPTA inhaler. Each inhaler contains 30 doses (60 blisters in total) and is designed to deliver a dosage of 100mcg of fluticasone furoate and 25mcg of vilanterol. The product is sold in strips of 30 blisters each, with one strip containing 100mcg of fluticasone furoate, and the other strip containing 25mcg of vilanterol.*
Breo Ellipta 200 mcg-25mcg 30 dose carton - breo ellipta spl graphic 27
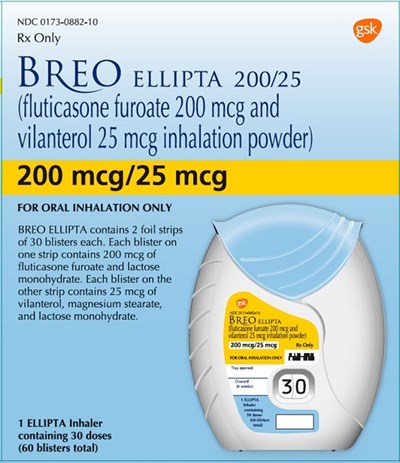
BREO ELLIPTA is a prescription medicine with the NDC code 0173-0882-10 for oral inhalation only. It contains two foil strips with 30 blisters each. One strip has blisters with 200 mcg of fluticasone furoate and lactose monohydrate, and the other strip has blisters with 25 mcg of vilanterol, magnesium stearate, and lactose monohydrate. It comes in an ELLIPTA inhaler containing 30 doses and 60 blisters in total. This medication is Rx only and is used for the treatment of respiratory diseases.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.