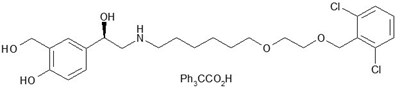
Product Images Trelegy Ellipta
View Photos of Packaging, Labels & Appearance
- FF chemical structure - trelegy ellipta spl graphic 01
- Umeclidinium chemical structure - trelegy ellipta spl graphic 02
- Vilanterol chemical structure - trelegy ellipta spl graphic 03
- Figure 1. Impact of Intrinsic Factors on the Pharmacokinetics (PK) of Fluticasone Furoate (FF) and Vilanterol (VI) Following Administration as Fluticasone Furoate/Vilanterol Combination - trelegy ellipta spl graphic 04
- Figure 2. Impact of Coadministered Drugsa on the Pharmacokinetics (PK) of Fluticasone Furoate (FF) and Vilanterol (VI) - trelegy ellipta spl graphic 05
- Figure 3. Impact of Intrinsic and Extrinsic Factors on the Systemic Exposure of Umeclidinium - trelegy ellipta spl graphic 06
- Trelegy Fig 4 - trelegy ellipta spl graphic 07
- Trelegy Fig 5 - trelegy ellipta spl graphic 08
- Figure 6. Least Squares (LS) Mean Change from Baseline in Trough FEV1 (mL) - trelegy ellipta spl graphic 09
- trelegy-ellipta-spl-graphic-10.jpg - trelegy ellipta spl graphic 10
- Figure A - trelegy ellipta spl graphic 11
- Figure B - trelegy ellipta spl graphic 12
- Figure C - trelegy ellipta spl graphic 13
- Figure D - trelegy ellipta spl graphic 14
- Figure E - trelegy ellipta spl graphic 15
- Figure F - trelegy ellipta spl graphic 16
- Figure G - trelegy ellipta spl graphic 17
- Figure H - trelegy ellipta spl graphic 18
- Figure I - trelegy ellipta spl graphic 19
- Figure J - trelegy ellipta spl graphic 20
- Figure K - trelegy ellipta spl graphic 21
- Figure L - trelegy ellipta spl graphic 22
- Trelegy Ellipta 30 dose carton - trelegy ellipta spl graphic 23
- trelegy ellipta spl graphic 24
- trelegy ellipta spl graphic 25
- trelegy ellipta spl graphic 26
- trelegy ellipta spl graphic 27
Product Label Images
The following 27 images provide visual information about the product associated with Trelegy Ellipta NDC 0173-0887 by Glaxosmithkline Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1. Impact of Intrinsic Factors on the Pharmacokinetics (PK) of Fluticasone Furoate (FF) and Vilanterol (VI) Following Administration as Fluticasone Furoate/Vilanterol Combination - trelegy ellipta spl graphic 04

Figure 2. Impact of Coadministered Drugsa on the Pharmacokinetics (PK) of Fluticasone Furoate (FF) and Vilanterol (VI) - trelegy ellipta spl graphic 05

Figure 3. Impact of Intrinsic and Extrinsic Factors on the Systemic Exposure of Umeclidinium - trelegy ellipta spl graphic 06

This text is a population description indicating recommendations for dose adjustments or caution when administering drugs for patients with severe renal or hepatic impairment. It also advises on the interaction of interacting drugs such as Ketoconazole and Verapamil with PK, AUC, Cmax and Crmax values, with fold changes or 90% confidence interval. The document also includes a chart showing changes relative to reference at different time intervals.*
Trelegy Fig 4 - trelegy ellipta spl graphic 07

This text lists different parameters related to the effect of certain factors in drug administration, such as renal and hepatic impairment, CYP2D6 inhibition, and P-gp transport. It also includes the recommended action in each case, which is generally to not adjust the dose.*
Trelegy Fig 5 - trelegy ellipta spl graphic 08

This appears to be a graph or chart displaying the LS Mean Change from Baseline in mL over time (in hours) for two different treatments - one with umeclidinium and fluticasone furoate/vilanterol and another with placebo and fluticasone furoate/vilanterol. The graph indicates that the treatment with umeclidinium and fluticasone furoate/vilanterol resulted in a greater change from baseline over time compared to the placebo and fluticasone furoate/vilanterol treatment. The specific context or purpose of this information is not available.*
Figure 6. Least Squares (LS) Mean Change from Baseline in Trough FEV1 (mL) - trelegy ellipta spl graphic 09

Figure A - trelegy ellipta spl graphic 11
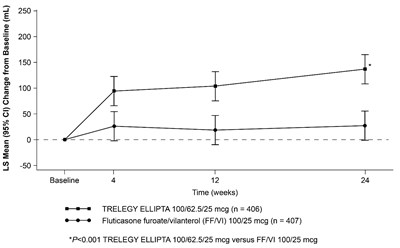
This is a graph comparing the change from baseline in mL of two medication treatments, TRELEGY ELLIPTA 100/62.5/25 mcg and Fluticasone furoate/vilanterol (FF/VI) 100/25 mcg, over the course of 24 weeks. The graph shows that TRELEGY ELLIPTA resulted in a significant increase in mL compared to FF/VI.*
Figure C - trelegy ellipta spl graphic 13

This description is not-available since it only lists the names of the items and does not provide any characteristics or properties about them.*
trelegy ellipta spl graphic 26

TRELEGY is a prescription medication that comes in inhalation powder form for oral inhalation only. It contains a combination of three active ingredients: fluticasone furoate, umeclidinium, and vilanterol. Each package contains two foil strips of 30 blisters each, providing a total of 30 doses or 60 blisters. The blisters on one strip contain 100 mcg of fluticasone furoate and lactose monohydrate, while the blisters on the other strip contain 62.5 mcg of umeclidinium, 25 mcg of vilanterol, magnesium stearate, and lactose monohydrate. The medication is intended for those with a prescription and the NDC number is 0173-0887-10.*
trelegy ellipta spl graphic 27

TRELEGY ELLIPTA is an inhalation powder medication that contains a combination of fluticasone furoate, umeclidinium, and vilanterol. It is used for oral inhalation only and comes with 2 strips of 30 blisters each, with each blister containing either 200 mg of fluticasone furoate and lactose monohydrate or 62.5 mcg of umeclidinium, 25 mcg of vilanterol, magnesium stearate, and lactose monohydrate. The package also includes an ELLIPTA inhaler with 30 doses (60 blisters total).*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.