FDA Label for Saponaria Officinalis
View Indications, Usage & Precautions
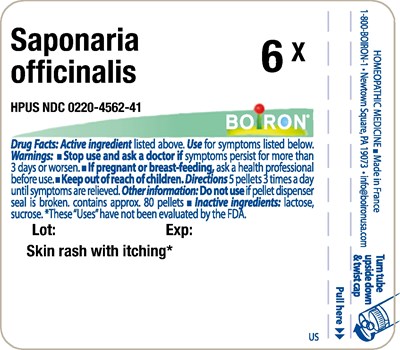
Saponaria Officinalis Product Label
The following document was submitted to the FDA by the labeler of this product Laboratoires Boiron. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Active Ingredients
Saponaria officinalis HPUS
6X, 9C
Use
Skin rash with itching*
Stop Use And Ask A Doctor If
symptoms persist for more than 3 days or worsen.
If Pregnant Or Breast-Feeding,
ask a health professional before use.
Directions
5 pellets 3 times a day until symptoms are relieved.
Other Information
Do not use if pellet dispenser seal is broken.
contains approx. 80 pellets
Inactive Ingredients
lactose, sucrose
Questions, Comments?
1-800-BOIRON-1
Newtown Square, PA 19073-3267
[email protected]
*
These "Uses" have not been evaluated by the FDA.
Principle Display Panel - Saponaria Officinalis Hpus 6X
* Please review the disclaimer below.