Product Images Gentamicin Sulfate In Sodium Chloride
View Photos of Packaging, Labels & Appearance
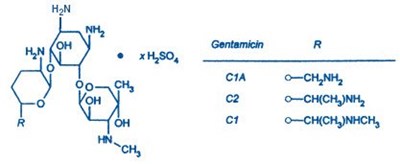
- Gentamicin Structural Formula - image 01
- Gentamicin Representative Container Label NDC 0338-0507-41 - image 02
- Gentamicin Serialization carton label 2B0851 - image 03
- Gentamicin Representative Container Label NDC 0338-0509-41 - image 04
- Gentamicin Serialization carton label 2B0852 - image 05
- Gentamicin Representative Container Label NDC 0338-0505-48 - image 06
- Gentamicin Serialization carton label 2B0863 - image 07
- Gentamicin Representative Container Label NDC 0338-0511-41 - image 08
- Gentamicin Serialization carton label 2B0853 - image 09
- Gentamicin Representative Carton Label NDC 0338-0503-48 - image 10
- Gentamicin Serialization carton label 2B0862 - image 11
- Gentamicin Representative Container Label 0338-0507-48 - image 12
- Gentamicin Representative Carton Label 0338-0507-48 - image 13
Product Label Images
The following 13 images provide visual information about the product associated with Gentamicin Sulfate In Sodium Chloride NDC 0338-0509 by Baxter Healthcare Corporation, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Gentamicin Representative Container Label NDC 0338-0507-41 - image 02

This is a description of Gentamicin Sulfate in 0.9% Sodium Chloride Injection, a sterile single dose container produced by Baxter Healthcare Corporation. It contains 60mg of Gentamicin Sulfate in every 50mL, and is meant for intravenous use only. The pH level may have been adjusted with either sodium hydroxide or sulfuric acid. The container should be stored at room temperature and precautions should be taken to avoid excessive heat. The dosing information can be found in the prescribing information.*
Gentamicin Serialization carton label 2B0851 - image 03

This is a description of a medication. The lot number T787987 and expiration date AUG 2025 are provided along with the product code 2B0851. The NDC code 0338-0507-41 is given for the medication, which contains Gentamicin Sulfate in 0.9% Sodium Chloride. It is an injection of 60 mg /50 mL. The remaining information appears to be a combination of codes, numbers, and dates that are likely not relevant to the user.*
Gentamicin Representative Container Label NDC 0338-0509-41 - image 04

This is a description of Gentamicin Sulfate Sodium Chloride Injection, which contains 80mg/50ml of gentamicin sulfate. It is intended for intravenous use only, and should not be combined with other medications. The pH may have been adjusted with sodium hydroxide or sulfuric acid. It is a sterile single-dose container that should be stored at room temperature, with precautions to avoid excessive heat. The prescribing information should be consulted for dosing. The manufacturer is Baxter Healthcare Corporation in Deerfield, IL, USA.*
Gentamicin Representative Container Label NDC 0338-0505-48 - image 06

This is a description of Gentamicin Sulfate in 0.9% Sodium Chloride Injection. It is a sterile single-dose container that comes in 100mg/100mL concentration. This injection is for intravenous use only and caution should be taken not to add supplementary medication. Each 100mL contains Gentamicin Sulfate USP and 900 mg of Sodium Chloride USP. The pH may have been adjusted with Sodium Hydroxide or Sulfuric Acid. Dosage information can be found in the prescribing information provided. It should be stored at room temperature but not exposed to excessive heat. The manufacturer is Baxter Healthcare Corporation in Deerfield, IL.*
Gentamicin Serialization carton label 2B0863 - image 07

The text contains a measurement value, "124100 mL", and some unreadable characters represented by "o" and "u". No useful description can be generated from this text.*
Gentamicin Representative Container Label NDC 0338-0511-41 - image 08

This is a description of a medication called Gentamicin Sulfate in 0.9% Sodium Chloride Injection. It comes in a 50 mL container, with 100 mg of the medication per 50 mL. It is for intravenous use only, and caution should be taken to maintain the unit in overwrap until ready for use. No supplementary medication should be added. The pH may have been adjusted with sodium hydroxide or sulfuric acid. The prescribing information should be consulted for dosing. The medication should be stored at room temperature and excessive heat should be avoided. Baxter Healthcare Corporation in Deerfield, IL, USA produced this sterile single dose container.*
Gentamicin Serialization carton label 2B0853 - image 09

This is a description of a medical injection containing Gentamicin Sulfate in 0.9% Sodium Chloride. It has a concentration of 100 mg per 50 mL, and the lot number is T123456. The expiration date is JAN 9999, and the product code is 2B0853. The NDC number is 0338.0511-41. However, there is some unclear text in the description.*
Gentamicin Representative Carton Label NDC 0338-0503-48 - image 10

This is a medication called Gentamicin Sulfate contained in a 100mL injection bottle with 80mg of Gentamicin Sulfate in 0.9% Sodium Chloride. It is meant for intravenous use only and should not be combined with supplementary medication. The medication should only be used when ready and stored at room temperature away from excessive heat. The bottle is sterile and for a single dose only. Further information about dosing should be obtained from the prescribing information. The manufacturer is Baxter Healthcare Corporation.*
Gentamicin Serialization carton label 2B0862 - image 11

Lot number T123456 with an expiration date of January 9999 contains Gentamicin Sulfate in 0.9% Sodium Chloride. It is an injection with 80mg/100mL strength, has a QrY value of 24400 mL, an NDC code of 0338.0503-48, and a code of 2B0862. The remaining text is not readable.*
Gentamicin Representative Container Label 0338-0507-48 - image 12

This is a description of a medication called Gentamicin Sulfate that comes in an injection form. The medication is mixed with sodium chloride and contains 120mg per 100mL or 1.2mg/mL. This medication is for intravenous use only and should be kept in the overwrap until needed; no supplementary medication should be added. The medication comes in a single dose container that is sterile. The container contains sodium chloride and may have its pH adjusted with sodium hydroxide or sulfuric acid. The container should be stored at room temperature without excessive heat. The details of administration and dosing can be found in the prescribing information. This medication is made by Baxter Healthcare Corporation in Illinois, USA.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.