FDA Label for Methylphenidate
View Indications, Usage & Precautions
- WARNING: DRUG DEPENDENCE
- 1 INDICATIONS AND USAGE
- 1.1 SPECIAL DIAGNOSTIC CONSIDERATIONS
- 1.2 NEED FOR COMPREHENSIVE TREATMENT PROGRAM
- 2 DOSAGE AND ADMINISTRATION
- 2.1 APPLICATION
- 2.2 REMOVAL OF METHYLPHENIDATE TRANSDERMAL SYSTEM
- 2.3 DISPOSAL OF METHYLPHENIDATE TRANSDERMAL SYSTEM
- 2.4 MAINTENANCE/EXTENDED TREATMENT
- 2.5 DOSE/WEAR TIME REDUCTION AND DISCONTINUATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4.1 HYPERSENSITIVITY TO METHYLPHENIDATE
- 4.2 AGITATION
- 4.3 GLAUCOMA
- 4.4 TICS
- 4.5 MONOAMINE OXIDASE INHIBITORS
- CHILDREN AND ADOLESCENTS
- ADULTS
- HYPERTENSION AND OTHER CARDIOVASCULAR CONDITIONS
- ASSESSING CARDIOVASCULAR STATUS IN PATIENTS BEING TREATED WITH STIMULANT MEDICATIONS
- PRE-EXISTING PSYCHOSIS
- BIPOLAR ILLNESS
- EMERGENCE OF NEW PSYCHOTIC OR MANIC SYMPTOMS
- AGGRESSION
- 5.3 SEIZURES
- 5.4 PRIAPISM
- 5.5 PERIPHERAL VASCULOPATHY, INCLUDING RAYNAUD’S PHENOMENON
- 5.6 LONG-TERM SUPPRESSION OF GROWTH
- 5.7 CHEMICAL LEUKODERMA
- 5.8 CONTACT SENSITIZATION
- 5.9 VISUAL DISTURBANCE
- 5.10 PATIENTS USING EXTERNAL HEAT
- 5.11 HEMATOLOGIC MONITORING
- 6 ADVERSE REACTIONS
- 6.1 CLINICAL TRIALS EXPERIENCE
- ADVERSE REACTIONS ASSOCIATED WITH DISCONTINUATION OF TREATMENT
- SKIN IRRITATION AND APPLICATION SITE REACTIONS
- MOST COMMONLY REPORTED ADVERSE REACTIONS
- ADVERSE REACTIONS WITH THE LONG-TERM USE OF METHYLPHENIDATE TRANSDERMAL SYSTEM
- 6.2 POSTMARKETING EXPERIENCE
- 6.3 ADVERSE REACTIONS WITH ORAL METHYLPHENIDATE PRODUCTS
- 7.1 MONOAMINE OXIDASE INHIBITORS (MAOI)
- 7.2 VASOPRESSOR AGENTS
- 7.3 ANTIHYPERTENSIVE AGENTS
- 7.4 COUMARIN ANTICOAGULANTS, ANTIDEPRESSANTS, AND SELECTIVE SEROTONIN REUPTAKE INHIBITORS
- 7.5 RISPERIDONE
- PREGNANCY EXPOSURE REGISTRY
- RISK SUMMARY
- FETAL/NEONATAL ADVERSE REACTIONS
- ANIMAL DATA
- CLINICAL CONSIDERATIONS
- 8.4 PEDIATRIC USE
- LONG TERM SUPPRESSION OF GROWTH
- JUVENILE ANIMAL TOXICITY DATA
- 8.5 GERIATRIC USE
- 9.1 CONTROLLED SUBSTANCE
- 9.2 ABUSE
- 9.3 DEPENDENCE
- 10.1 SIGNS AND SYMPTOMS
- 10.2 RECOMMENDED TREATMENT
- 10.3 POISON CONTROL CENTER
- 11 DESCRIPTION
- 11.1 TRANSDERMAL SYSTEM COMPONENTS
- 12.1 MECHANISM OF ACTION
- 12.2 PHARMACODYNAMICS
- 12.3 PHARMACOKINETICS
- ABSORPTION
- DOSE PROPORTIONALITY
- DISTRIBUTION
- METABOLISM AND EXCRETION
- FOOD EFFECTS
- GENDER
- RACE
- AGE
- RENAL IMPAIRMENT
- HEPATIC IMPAIRMENT
- CARCINOGENESIS
- MUTAGENESIS
- IMPAIRMENT OF FERTILITY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- MEDICATION GUIDE
- PRINCIPAL DISPLAY PANEL – 1.1 MG/HR
- PRINCIPAL DISPLAY PANEL – 1.6 MG/HOUR
- PRINCIPAL DISPLAY PANEL – 2.2 MG/HOUR
- PRINCIPAL DISPLAY PANEL – 3.3 MG/HOUR
Methylphenidate Product Label
The following document was submitted to the FDA by the labeler of this product Mylan Pharmaceuticals Inc.. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Warning: Drug Dependence
Methylphenidate transdermal system should be given cautiously to patients with a history of drug dependence or alcoholism. Chronic abusive use can lead to marked tolerance and psychological dependence with varying degrees of abnormal behavior. Frank psychotic episodes can occur, especially with parenteral abuse. Careful supervision is required during withdrawal from abusive use, since severe depression may occur. Withdrawal following chronic therapeutic use may unmask symptoms of the underlying disorder that may require follow-up.
1 Indications And Usage
Methylphenidate transdermal system is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD).
The efficacy of methylphenidate transdermal system in patients diagnosed with ADHD was established in two 7-week controlled clinical trials in children (ages 6-12) and one 7-week, controlled clinical trial in adolescents (ages 13-17).
A diagnosis of ADHD (DSM-IV-TR®) implies the presence of hyperactive-impulsive or inattentive symptoms that caused impairment and were present before age 7 years. The symptoms must cause clinically significant impairment, e.g., in social, academic, or occupational functioning, and be present in two or more settings, e.g., school (or work) and at home. The symptoms must not be better accounted for by another mental disorder. For the Inattentive Type, at least six of the following symptoms must have persisted for at least 6 months: lack of attention to details/careless mistakes; lack of sustained attention; poor listener; failure to follow through on tasks; poor organization; avoids tasks requiring sustained mental effort; loses things; easily distracted; forgetful. For the Hyperactive-Impulsive Type, at least six of the following symptoms must have persisted for at least 6 months: fidgeting/squirming; leaving seat; inappropriate running/climbing; difficulty with quiet activities; “on the go;” excessive talking; blurting answers; can’t wait turn; intrusive. The Combined Type requires both inattentive and hyperactive-impulsive criteria to be met.
1.1 Special Diagnostic Considerations
The specific etiology of this syndrome is unknown, and there is no single diagnostic test. Adequate diagnosis requires the use not only of medical but of special psychological, educational, and social resources. Learning may or may not be impaired. The diagnosis must be based upon a complete history and evaluation of the patient and not solely on the presence of the required number of DSM-IV-TR® characteristics.
1.2 Need For Comprehensive Treatment Program
Methylphenidate transdermal system is indicated as an integral part of a total treatment program for ADHD that may include other measures (psychological, educational, social) for patients with this syndrome. Drug treatment may not be indicated for all patients with this syndrome. Stimulants are not intended for use in the patient who exhibits symptoms secondary to environmental factors and/or other primary psychiatric disorders, including psychosis. Appropriate educational placement is essential and psychosocial intervention is often helpful. When remedial measures alone are insufficient, the decision to prescribe stimulant medication will depend upon the physician’s assessment of the chronicity and severity of the patient’s symptoms.
2 Dosage And Administration
It is recommended that methylphenidate transdermal system be applied to the hip area 2 hours before an effect is needed and should be removed 9 hours after application. Dosage should be titrated to effect. The recommended dose titration schedule is shown in the table below. Dose titration, final dosage, and wear time should be individualized according to the needs and response of the patient.
Upward Titration, if Response is Not Maximized | |||||||
Week 1 | Week 2 | Week 3 | Week 4 | ||||
Transdermal System Size | 9.6 cm² | 14.4 cm² | 19.2 cm² | 28.8 cm² | |||
Nominal Delivered Dose Nominal in vivo delivery rate in children and adolescents when applied to the hip, based on a 9-hour wear period. (mg/9 hours) | 10 mg | 15 mg | 20 mg | 30 mg | |||
Delivery Rate | (1.1 mg/hr) | (1.6 mg/hr) | (2.2 mg/hr) | (3.3 mg/hr) | |||
Patients converting from another formulation of methylphenidate should follow the above titration schedule due to differences in bioavailability of methylphenidate transdermal system compared to other products.
2.1 Application
The parent or caregiver should be encouraged to use the administration chart included with each carton of methylphenidate transdermal system to monitor application and removal time, and method of disposal. It is recommended that parents or caregivers apply and remove the transdermal system for children; responsible adolescents may apply or remove the transdermal system themselves if appropriate. The Medication Guide included at the end of this insert also includes a timetable to calculate when to remove methylphenidate transdermal system, based on the 9-hour application time.
The adhesive side of methylphenidate transdermal system should be placed on a clean, dry area of the hip. The area selected should not be oily, damaged, or irritated. Apply the transdermal system to the hip area avoiding the waistline, since clothing may cause the transdermal system to rub off. When applying the transdermal system the next morning, place on the opposite hip at a new site if possible.
If patients or caregivers experience difficulty separating the transdermal system from the release liner or observe transfer of adhesive to the liner, tearing and/or other damage to the transdermal system during removal from the liner, the transdermal system should be discarded according to the directions provided below, and a new transdermal system should be applied. Patients or caregivers should inspect the release liner to ensure that no adhesive containing medication has transferred to the liner. If adhesive transfer has occurred, the transdermal system should be discarded.
Methylphenidate transdermal system should be applied immediately after opening the individual pouch and removing the protective liner. Do not use if the individual pouch seal is broken or if the transdermal system appears to be damaged. Do not cut transdermal systems. Only intact transdermal systems should be applied. The transdermal system should then be pressed firmly in place with the palm of the hand for approximately 30 seconds, making sure that there is good contact of the transdermal system with the skin, especially around the edges. Exposure to water during bathing, swimming, or showering can affect transdermal system adherence. Transdermal systems should not be applied or re-applied with dressings, tape, or other common adhesives. In the event that a transdermal system does not fully adhere to the skin upon application, or becomes partially or fully detached during wear time, the transdermal system should be discarded according to the directions provided in this label [see Dosage and Administration (2.3)] and a new transdermal system may be applied at a different site. The total recommended wear time for that day should remain 9 hours regardless of the number of transdermal systems used [see Patient Counseling Information (17)].
All patients should be advised to avoid exposing the methylphenidate transdermal system application site to direct external heat sources, such as hair dryers, heating pads, electric blankets, heated water beds, etc., while wearing the transdermal system [see Warnings and Precautions (5.10)]. When heat is applied to methylphenidate transdermal system after transdermal system application, both the rate and the extent of absorption are significantly increased. The temperature-dependent increase in methylphenidate absorption can be greater than 2-fold [see Clinical Pharmacology (12.3)]. This increased absorption can be clinically significant and result in overdose of methylphenidate [see Overdosage (10)].
Transdermal systems should not be stored in refrigerators or freezers.
2.2 Removal Of Methylphenidate Transdermal System
Methylphenidate transdermal systems should be peeled off slowly. If necessary, transdermal system removal may be facilitated by gently applying an oil-based product (i.e., petroleum jelly, olive oil, or mineral oil) to the transdermal system edges, gently working the oil underneath the transdermal system edges. If any adhesive remains on the skin following transdermal system removal, an oil-based product may be applied to transdermal system sites in an effort to gently loosen and remove any residual adhesive that remains following transdermal system removal.
In the unlikely event that a transdermal system remains tightly adhered despite these measures, the patient or caregiver should contact the physician or pharmacist. Nonmedical adhesive removers and acetone-based products (i.e., nail polish remover) should not be used to remove methylphenidate transdermal systems or adhesive.
2.3 Disposal Of Methylphenidate Transdermal System
Upon removal of methylphenidate transdermal system, used transdermal systems should be folded so that the adhesive side of the transdermal system adheres to itself and should be flushed down the toilet or disposed of in an appropriate lidded container. If the patient stops using the prescription, each unused transdermal system should be removed from its individual pouch, separated from the protective liner, folded onto itself, and disposed of in the same manner as used transdermal systems.
The parent or caregiver should be encouraged to record on the administration chart included with each carton the time that each transdermal system was applied and removed. If a transdermal system was removed without the parent or caregiver’s knowledge, or if a transdermal system is missing from the carton or pouch, the parent or caregiver should be encouraged to ask the child when and how the transdermal system was removed.
2.4 Maintenance/Extended Treatment
There is no body of evidence available from controlled clinical trials to indicate how long the patient with ADHD should be treated with methylphenidate transdermal system. It is generally agreed, however, that pharmacological treatment of ADHD may be needed for extended periods. The effectiveness of methylphenidate transdermal system for long-term use, i.e., for more than 7 weeks, has not been systematically evaluated in controlled trials. The physician who elects to use methylphenidate transdermal system for extended periods should periodically re-evaluate the long-term usefulness of methylphenidate transdermal system for the individual patient with periods off medication to assess the patient’s functioning without pharmacotherapy. Improvement may be sustained when the drug is either temporarily or permanently discontinued.
2.5 Dose/Wear Time Reduction And Discontinuation
Methylphenidate transdermal system may be removed earlier than 9 hours if a shorter duration of effect is desired or late day side effects appear. Plasma concentrations of d-methylphenidate generally begin declining when the transdermal system is removed, although absorption may continue for several hours. Individualization of wear time may help manage some of the side effects caused by methylphenidate. If aggravation of symptoms or other adverse events occur, the dosage or wear time should be reduced, or, if necessary, the drug should be discontinued. Residual methylphenidate remains in used transdermal systems when worn as recommended.
3 Dosage Forms And Strengths
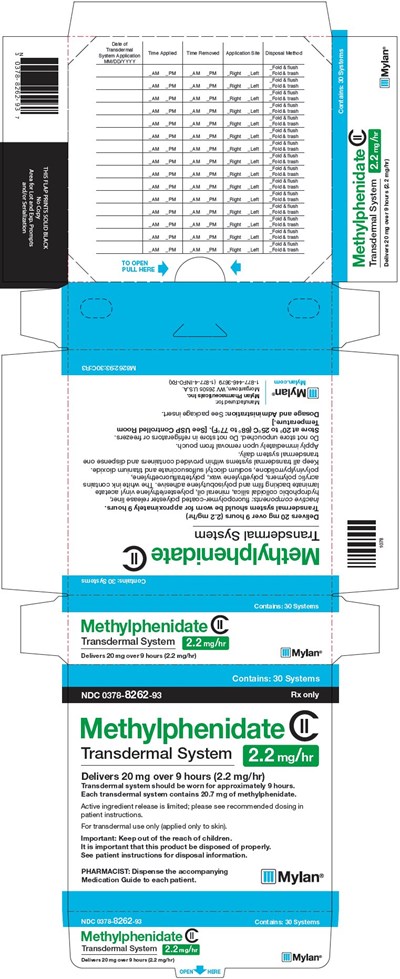
Four dosage strengths of Methylphenidate Transdermal System are available as 10 mg/9 hrs (1.1 mg/hr), 15 mg/9 hrs (1.6 mg/hr), 20 mg/9 hrs (2.2 mg/hr) or 30 mg/9 hrs (3.3 mg/hr) of methylphenidate.
• Each dosage form is a translucent rectangular transdermal system with rounded corners consisting of a matte backing film randomly printed with “Methylphenidate Transdermal System” and the “mg/hr” strength in white ink, an adhesive layer and a clear to slightly hazy oversized release liner that is slit. Each transdermal system is overlaid with an additional clear to slightly hazy oversized release liner and is contained in a square, flat pouch that is imprinted with lot number and expiration date.
Nominal Dose Delivered (mg) Over 9 Hours Nominal in vivo delivery rate in children and adolescents when applied to the hip, based on a 9-hour wear period. | Dosage Rate | Transdermal System Size | Methylphenidate Content per Transdermal System (mg) |
10 | 1.1 | 9.6 | 10.4 |
15 | 1.6 | 14.4 | 15.6 |
20 | 2.2 | 19.2 | 20.7 |
30 | 3.3 | 28.8 | 31.1 |
4.1 Hypersensitivity To Methylphenidate
Methylphenidate transdermal system is contraindicated in patients known to be hypersensitive to methylphenidate or other components of the product (fluoropolymer-coated polyester, hydrophobic colloidal silica, mineral oil, polyester/ethylene vinyl acetate laminate film backing, polyisobutylene adhesive and white ink). The white ink contains acrylic polymers, polyethylene wax, polytetrafluoroethylene, polyvinylpyrrolidone, sodium dioctyl sulfosuccinate and titanium dioxide [see Description (11.1)].
4.2 Agitation
Methylphenidate transdermal system is contraindicated in patients with marked anxiety, tension, and agitation, since the drug may aggravate these symptoms.
4.3 Glaucoma
Methylphenidate transdermal system is contraindicated in patients with glaucoma.
4.4 Tics
Methylphenidate transdermal system is contraindicated in patients with motor tics or with a family history or diagnosis of Tourette’s syndrome [see Adverse Reactions (6.1)].
4.5 Monoamine Oxidase Inhibitors
Methylphenidate transdermal system is contraindicated during treatment with monoamine oxidase inhibitors, and also within a minimum of 14 days following discontinuation of treatment with a monoamine oxidase inhibitor (hypertensive crises may result).
Children And Adolescents
Sudden death has been reported in association with CNS stimulant treatment at usual doses in children and adolescents with structural cardiac abnormalities or other serious heart problems. Although some serious heart problems alone carry an increased risk of sudden death, stimulant products generally should not be used in children or adolescents with known serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or other serious cardiac problems that may place them at increased vulnerability to the sympathomimetic effects of a stimulant drug.
Adults
Sudden deaths, stroke, and myocardial infarction have been reported in adults taking stimulant drugs at usual doses for ADHD. Although the role of stimulants in these adult cases is also unknown, adults have a greater likelihood than children of having serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other serious cardiac problems. Adults with such abnormalities should also generally not be treated with stimulant drugs.
Hypertension And Other Cardiovascular Conditions
Stimulant medications cause a modest increase in average blood pressure (about 2-4 mmHg) and average heart rate (about 3-6 bpm), and individuals may have larger increases. While the mean changes alone would not be expected to have short-term consequences, all patients should be monitored for larger changes in heart rate and blood pressure. Caution is indicated in treating patients whose underlying medical conditions might be compromised by increases in blood pressure or heart rate, e.g., those with pre-existing hypertension, heart failure, recent myocardial infarction, or ventricular arrhythmia [see Adverse Reactions (6.1)].
Assessing Cardiovascular Status In Patients Being Treated With Stimulant Medications
Children, adolescents, or adults who are being considered for treatment with stimulant medications should have a careful history (including assessment for a family history of sudden death or ventricular arrhythmia) and physical exam to assess for the presence of cardiac disease, and should receive further cardiac evaluation if findings suggest such disease (e.g., electrocardiogram and echocardiogram). Patients who develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease during stimulant treatment should undergo a prompt cardiac evaluation.
Pre-Existing Psychosis
Administration of stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a pre-existing psychotic disorder.
Bipolar Illness
Particular care should be taken in using stimulants to treat ADHD in patients with comorbid bipolar disorder because of concern for possible induction of a mixed/manic episode in such patients. Prior to initiating treatment with a stimulant, patients with comorbid depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression.
Emergence Of New Psychotic Or Manic Symptoms
Treatment emergent psychotic or manic symptoms, e.g., hallucinations, delusional thinking, or mania in children and adolescents without a prior history of psychotic illness or mania can be caused by stimulants at usual doses. If such symptoms occur, consideration should be given to a possible causal role of the stimulant, and discontinuation of treatment may be appropriate. In a pooled analysis of multiple short term, placebo-controlled studies, such symptoms occurred in about 0.1% (4 patients with events out of 3,482 exposed to methylphenidate or amphetamine for several weeks at usual doses) of stimulant-treated patients compared to none in placebo-treated patients.
Aggression
Aggressive behavior or hostility is often observed in children and adolescents with ADHD, and has been reported in clinical trials and the postmarketing experience of some medications indicated for the treatment of ADHD. Although there is no systematic evidence that stimulants cause aggressive behavior or hostility, patients beginning treatment for ADHD should be monitored for the appearance of or worsening of aggressive behavior or hostility.
5.3 Seizures
There is some clinical evidence that stimulants may lower the convulsive threshold in patients with prior history of seizures, in patients with prior EEG abnormalities in absence of seizures, and, very rarely, in patients without a history of seizures and no prior EEG evidence of seizures. In the presence of seizures, the drug should be discontinued.
5.4 Priapism
Prolonged and painful erections, sometimes requiring surgical intervention, have been reported with methylphenidate products in both pediatric and adult patients. Priapism was not reported with drug initiation but developed after some time on the drug, often subsequent to an increase in dose. Priapism has also appeared during a period of drug withdrawal (drug holidays or during discontinuation). Patients who develop abnormally sustained or frequent and painful erections should seek immediate medical attention.
5.5 Peripheral Vasculopathy, Including Raynaud’S Phenomenon
Stimulant medications, including methylphenidate transdermal system, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud’s phenomenon. Signs and symptoms are usually intermittent and mild; however, very rare sequelae include digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud’s phenomenon, were observed in post-marketing reports at different times and at therapeutic doses in all age groups throughout the course of treatment. Signs and symptoms generally improve after reduction in dose or discontinuation of drug. Careful observation for digital changes is necessary during treatment with ADHD stimulants. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
5.6 Long-Term Suppression Of Growth
Careful follow-up of weight and height in children ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated children over 36 months (to the ages of 10 to 13 years), suggests that consistently medicated children (i.e., treatment for 7 days per week throughout the year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this period of development. Published data are inadequate to determine whether chronic use of amphetamines may cause a similar suppression of growth, however, it is anticipated that they likely have this effect as well. Therefore, growth should be monitored during treatment with stimulants, and patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted.
5.7 Chemical Leukoderma
Methylphenidate transdermal system use may result in a persistent loss of skin pigmentation at and around the application site. Loss of pigmentation, in some cases, has been reported at other sites distant from the application site. Chemical leukoderma can mimic the appearance of vitiligo, particularly when the loss of skin pigmentation involves areas distant from the application site. Individuals with a history of vitiligo and/or a family history of vitiligo may be more at risk. Skin depigmentation may persist even after methylphenidate transdermal system use is discontinued. Monitor for signs of skin depigmentation, and advise patients to immediately inform their healthcare provider if changes in skin pigmentation occur. Discontinue use of the methylphenidate transdermal system in patients with chemical leukoderma.
5.8 Contact Sensitization
In an open-label study of 305 subjects conducted to characterize dermal reactions in children with ADHD treated with methylphenidate transdermal system using a 9-hour wear time, one subject (0.3%) was confirmed by transdermal system testing to be sensitized to methylphenidate (allergic contact dermatitis). This subject experienced erythema and edema at methylphenidate transdermal system application sites with concurrent urticarial lesions on the abdomen and legs resulting in treatment discontinuation. This subject was not transitioned to oral methylphenidate.
Use of methylphenidate transdermal system may lead to contact sensitization. Methylphenidate transdermal system should be discontinued if contact sensitization is suspected. Erythema is commonly seen with use of methylphenidate transdermal system and is not by itself an indication of sensitization. However, contact sensitization should be suspected if erythema is accompanied by evidence of a more intense local reaction (edema, papules, vesicles) that does not significantly improve within 48 hours or spreads beyond the transdermal system site. Confirmation of a diagnosis of contact sensitization (allergic contact dermatitis) may require further diagnostic testing.
Patients sensitized from use of methylphenidate transdermal system, as evidenced by development of an allergic contact dermatitis, may develop systemic sensitization or other systemic reactions if methylphenidate-containing products are taken via other routes, e.g., orally. Manifestations of systemic sensitization may include a flare-up of previous dermatitis or of prior positive transdermal system-test sites, or generalized skin eruptions in previously unaffected skin. Other systemic reactions may include headache, fever, malaise, arthralgia, diarrhea, or vomiting. No cases of systemic sensitization have been observed in clinical trials of methylphenidate transdermal system.
Patients who develop contact sensitization to methylphenidate transdermal system and require oral treatment with methylphenidate should be initiated on oral medication under close medical supervision. It is possible that some patients sensitized to methylphenidate by exposure to methylphenidate transdermal system may not be able to take methylphenidate in any form.
5.9 Visual Disturbance
Difficulties with accommodation and blurring of vision have been reported with stimulant treatment.
5.10 Patients Using External Heat
Patients should be advised to avoid exposing the methylphenidate transdermal system application site to direct external heat sources, such as hair dryers, heating pads, electric blankets, heated water beds, etc., while wearing the transdermal system. When heat is applied to methylphenidate transdermal system after transdermal system application, both the rate and extent of absorption are significantly increased. The temperature-dependent increase in methylphenidate absorption can be greater than 2-fold [see Clinical Pharmacology (12.3)]. This increased absorption can be clinically significant and can result in overdose of methylphenidate [see Overdosage (10)].
5.11 Hematologic Monitoring
Periodic CBC, differential, and platelet counts are advised during prolonged therapy.
6 Adverse Reactions
Detailed information on serious and adverse reactions of particular importance is provided in the Boxed Warning and Warnings and Precautions (5) sections:
• Drug dependence [see Boxed Warning]• Hypersensitivity to Methylphenidate [see Contraindications (4.1)]• Marked anxiety, tension, or agitation [see Contraindications (4.2)]• Glaucoma [see Contraindications (4.3)]• Tics or a family history of Tourette’s syndrome [see Contraindications (4.4)]• Monoamine Oxidase Inhibitors [see Contraindications (4.5) and Drug Interactions (7.1)]• Serious Cardiovascular Events [see Warnings and Precautions (5.1)]• Increase in Blood Pressure [see Warnings and Precautions (5.1)]• Psychiatric Adverse Events [see Warnings and Precautions (5.2)]• Seizures [see Warnings and Precautions (5.3)]• Priapism [see Warnings and Precautions (5.4)]• Peripheral Vasculopathy [see Warnings and Precautions (5.5)]• Long-Term Suppression of Growth [see Warnings and Precautions (5.6)]• Chemical Leukoderma [see Warnings and Precautions (5.7)]• Contact Sensitization [see Warnings and Precautions (5.8)]• Visual Disturbance [see Warnings and Precautions (5.9)]• External Heat [see Warnings and Precautions (5.10)]• Hematologic Monitoring [see Warnings and Precautions (5.11)]
The most commonly reported (frequency ≥ 5% and twice the rate of placebo) adverse reactions in a controlled trial in children aged 6-12 included appetite decreased, insomnia, nausea, vomiting, weight decreased, tic, affect lability, and anorexia. The most commonly reported (frequency ≥ 5% and twice the rate of placebo) adverse reactions in a controlled trial in adolescents aged 13-17 were appetite decreased, nausea, insomnia, weight decreased, dizziness, abdominal pain, and anorexia [see Adverse Reactions (6.1)].
The most common (≥ 2% of subjects) adverse reaction associated with discontinuations in double-blind clinical trials in children or adolescents was application site reactions [see Adverse Reactions (6.1)].
The overall methylphenidate transdermal system development program included exposure to methylphenidate transdermal system in a total of 2,152 participants in clinical trials, including 1,529 children aged 6-12, 223 adolescents aged 13-17, and 400 adults. The 1,752 child and adolescent subjects aged 6-17 years were evaluated in 10 controlled clinical studies, 7 open-label clinical studies, and 5 clinical pharmacology studies. In a combined studies pool of children using methylphenidate transdermal system with a wear time of 9 hours, 212 subjects were exposed for ≥ 6 months and 115 were exposed for ≥ 1 year; 85 adolescents have been exposed for ≥ 6 months. Most patients studied were exposed to methylphenidate transdermal system sizes of 12.5 cm², 18.75 cm², 25 cm², or 37.5 cm², with a wear time of 9 hours.
In the data presented below, the adverse reactions reported during exposure were obtained primarily by general inquiry at each visit, and were recorded by the clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse reactions without first grouping similar types of events into a smaller number of standardized event categories.
Throughout this section adverse reactions reported are events that were considered to be reasonably associated with the use of methylphenidate transdermal system based on comprehensive assessment of the available adverse event information. A causal association for methylphenidate transdermal system often cannot be reliably established in individual cases. Further, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions Associated With Discontinuation Of Treatment
In a 7-week double-blind, parallel-group, placebo-controlled study in children with ADHD conducted in the outpatient setting, 7.1% (7/98) of patients treated with methylphenidate transdermal system discontinued due to adverse events compared with 1.2% (1/85) receiving placebo. The most commonly reported (≥ 1% and twice the rate of placebo) adverse reactions leading to discontinuation in the methylphenidate transdermal system group were application site reaction (2%), tics (1%), headache (1%), and irritability (1%).
In a 7-week double-blind, parallel-group, placebo-controlled study in adolescents with ADHD conducted in the outpatient setting, 5.5% (8/145) of patients treated with methylphenidate transdermal system discontinued due to adverse reactions compared with 2.8% (2/72) receiving placebo. The most commonly reported adverse reactions leading to discontinuation in the methylphenidate transdermal system group were application site reaction (2%) and decreased appetite/anorexia (1.4%).
Skin Irritation And Application Site Reactions
Methylphenidate transdermal system is a dermal irritant. In addition to the most commonly reported adverse reactions presented in Table 2, the majority of subjects in those studies had minimal to definite skin erythema at the transdermal system application site. This erythema generally caused no or minimal discomfort and did not usually interfere with therapy or result in discontinuation from treatment. Erythema is not by itself a manifestation of contact sensitization. However, contact sensitization should be suspected if erythema is accompanied by evidence of a more intense local reaction (edema, papules, vesicles) that does not significantly improve within 48 hours or spreads beyond the transdermal system site [see Warnings and Precautions (5.8)].
Most Commonly Reported Adverse Reactions
Table 2 lists treatment-emergent adverse reactions reported in ≥ 1% methylphenidate transdermal system-treated children or adolescents with ADHD in two 7 week double-blind, parallel-group, placebo-controlled studies conducted in the outpatient setting. Overall, in these studies, 75.5% of children and 78.6% of adolescents experienced at least 1 adverse event.
Adolescents | Children | |||
System Organ Class | Placebo | Methylphenidate Transdermal System | Placebo | Methylphenidate Transdermal System |
Preferred term | N = 72 | N = 145 | N = 85 | N = 98 |
Cardiac Disorders | ||||
Tachycardia | 0 (0) | 1 (0.7) | 0 (0) | 1 (1.0) |
Gastrointestinal disorders | ||||
Abdominal pain | 0 (0) | 7 (4.8) | 5 (5.9) | 7 (7.1) |
Nausea | 2 (2.8) | 14 (9.7) | 2 (2.4) | 12 (12.2) |
Vomiting | 1 (1.4) | 5 (3.4) | 4 (4.7) | 10 (10.2) |
Investigations | ||||
Weight decreased | 1 (1.4) | 8 (5.5) | 0 (0) | 9 (9.2) |
Metabolism and nutrition disorders | ||||
Anorexia | 1 (1.4) | 7 (4.8) | 1 (1.2) | 5 (5.1) |
Decreased appetite | 1 (1.4) | 37 (25.5) | 4 (4.7) | 25 (25.5) |
Nervous system disorders | ||||
Dizziness | 1 (1.4) | 8 (5.5) | 1 (1.2) | 0 (0) |
Headache | 9 (12.5) | 18 (12.4) | 10 (11.8) | 15 (15.3) |
Psychiatric disorders | ||||
Affect lability | 1 (1.4) | 0 (0) | 0 (0) | 6 (6.1) Six subjects had affect lability, all judged as mild and described as increased emotionally sensitive, emotionality, emotional instability, emotional lability, and intermittent emotional |
Insomnia | 2 (2.8) | 9 (6.2) | 4 (4.7) | 13 (13.3) |
Irritability | 5 (6.9) | 16 (11) | 4 (4.7) | 7 (7.1) |
Tic | 0 (0) | 0 (0) | 0 (0) | 7 (7.1) |
Adverse Reactions With The Long-Term Use Of Methylphenidate Transdermal System
In a long-term open-label study of up to 12 months duration in 326 children wearing methylphenidate transdermal system 9 hours daily, the most common (≥ 10%) adverse reactions were decreased appetite, headache, and weight decreased. A total of 30 subjects (9.2%) were withdrawn from the study due to adverse events and 22 additional subjects (6.7%) discontinued treatment as the result of an application site reaction. Other than application site reactions, affect lability (5 subjects, 1.5%) was the only additional adverse reaction leading to discontinuation reported with a frequency of greater than 1%.
In a long-term open-label study of up to 6 months duration in 162 adolescents wearing methylphenidate transdermal system 9 hours daily, the most common (≥ 10%) adverse reactions were decreased appetite and headache. A total of 9 subjects (5.5%) were withdrawn from the study due to adverse events and 3 additional subjects (1.9%) discontinued treatment as the result of an application site reaction. Other adverse reactions leading to discontinuation that occurred with a frequency of greater than 1% included affect lability and irritability (2 subjects each, 1.2%).
6.2 Postmarketing Experience
In addition, the following adverse reactions have been identified during the postapproval use of methylphenidate transdermal system. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to methylphenidate transdermal system exposure.
Cardiac Disorders: palpitations
Eye Disorders: visual disturbances, blurred vision, mydriasis, accommodation disorder
General Disorders and Administration Site Disorders: fatigue, application site reactions such as bleeding, bruising, burn, burning, dermatitis, discharge, discoloration, discomfort, dryness, eczema, edema, erosion, erythema, excoriation, exfoliation, fissure, hyperpigmentation, hypopigmentation, induration, infection, inflammation, irritation, pain, papules, paresthesia, pruritus, rash, scab, swelling, ulcer, urticaria, vesicles, and warmth.
Immune System Disorders: hypersensitivity reactions including generalized erythematous and urticarial rashes, allergic contact dermatitis, angioedema, and anaphylaxis
Investigations: blood pressure increased
Nervous System Disorders: convulsion, dyskinesia, lethargy, somnolence, serotonin syndrome in combination with serotonergic drugs, and extrapyramidal disorder
Psychiatric Disorders: depression, hallucination, nervousness, and libido changes
Skin and Subcutaneous Tissue Disorders: alopecia
6.3 Adverse Reactions With Oral Methylphenidate Products
Nervousness and insomnia are the most common adverse reactions reported with other methylphenidate products. In children, loss of appetite, abdominal pain, weight loss during prolonged therapy, insomnia, and tachycardia may occur more frequently; however, any of the other adverse reactions listed below may also occur.
Other reactions include:
Cardiac Disorders: angina, arrhythmia, and pulse increased or decreased
Immune System Disorders: hypersensitivity reactions including skin rash, urticaria, fever, arthralgia, exfoliative dermatitis, erythema multiforme with histopathological findings of necrotizing vasculitis, and thrombocytopenic purpura
Metabolism and Nutrition Disorders: anorexia and weight loss during prolonged therapy
Nervous System Disorders: drowsiness, rare reports of Tourette’s syndrome and toxic psychosis.
Very rare reports of neuroleptic malignant syndrome (NMS) have been received, and, in most of these, patients were concurrently receiving therapies associated with NMS. In a single report, a ten-year-old boy who had been taking methylphenidate for approximately 18 months experienced an NMS-like event within 45 minutes of ingesting his first dose of venlafaxine. It is uncertain whether this case represented a drug-drug interaction, a response to either drug alone, or some other cause.
Vascular Disorders: blood pressure increased or decreased and cerebral arteritis and/or occlusion
Although a definite causal relationship has not been established, the following have been reported in patients taking methylphenidate:
Blood/Lymphatic System Disorders: leukopenia and/or anemia
Hepatobiliary Disorders: abnormal liver function, ranging from transaminase elevation to severe hepatic injury
Psychiatric Disorders: transient depressed mood
Skin and Subcutaneous Tissue Disorders: scalp hair loss
Musculoskeletal and Connective Tissue Disorders: rhabdomyolysis
7.1 Monoamine Oxidase Inhibitors (Maoi)
Concomitant use of MAOIs and CNS stimulants, including methylphenidate transdermal system, can cause hypertensive crisis. Potential outcomes include death, stroke, myocardial infarction, aortic dissection, ophthalmological complications, eclampsia, pulmonary edema, and renal failure [see Contraindications (4.5)]. Concomitant use of methylphenidate transdermal system with MAOIs or within 14 days after discontinuing MAOI treatment is contraindicated.
7.2 Vasopressor Agents
Because of a possible effect on blood pressure, methylphenidate transdermal system should be used cautiously with pressor agents.
7.3 Antihypertensive Agents
Methylphenidate may decrease the effectiveness of drugs used to treat hypertension. Monitor blood pressure and adjust the dosage of the antihypertensive drug as needed [see Warnings and Precautions (5.1)].
7.4 Coumarin Anticoagulants, Antidepressants, And Selective Serotonin Reuptake Inhibitors
Human pharmacologic studies have shown that methylphenidate may inhibit the metabolism of coumarin anticoagulants, anticonvulsants (e.g., phenobarbital, phenytoin, primidone), and some tricyclic drugs (e.g., imipramine, clomipramine, desipramine) and selective serotonin reuptake inhibitors. Downward dose adjustments of these drugs may be required when given concomitantly with methylphenidate. It may be necessary to adjust the dosage and monitor plasma drug concentrations (or, in the case of coumarin, coagulation times), when initiating or discontinuing methylphenidate.
7.5 Risperidone
Combined use of methylphenidate with risperidone when there is a change in dosage, whether an increase or decrease, of either or both medications, may increase the risk of extrapyramidal symptoms (EPS). Monitor for signs of EPS.
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ADHD medications, including methylphenidate transdermal system, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for ADHD Medications at 1-866-961-2388 or visit https://womensmentalhealth.org/adhd-medications/.
Risk Summary
Published studies and post-marketing reports on methylphenidate use during pregnancy are insufficient to identify a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. There are risks to the fetus associated with the use of central nervous system (CNS) stimulants during pregnancy (see Clinical Considerations).
No effects on morphological development were observed in embryo-fetal development studies with oral administration of methylphenidate to pregnant rats and rabbits during organogenesis. However, spina bifida was observed in rabbits when given oral doses of 200 mg/kg/day. When methylphenidate was administered orally to rats throughout pregnancy and lactation, offspring growth and survival were decreased at maternally toxic doses (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinical recognized pregnancies is 2-4% and 15-20%, respectively.
Limited published literature, based on breast milk sampling from five mothers, reports that methylphenidate is present in human milk, which resulted in infant doses of 0.16% to 0.7% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 1.1 and 2.7. There are no reports of adverse effects on the breastfed infant and no effects on milk production. Long-term neurodevelopmental effects on infants from stimulant exposure are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for methylphenidate transdermal system and any potential adverse effects on the breastfed infant from methylphenidate or from the underlying maternal condition.
Fetal/Neonatal Adverse Reactions
CNS stimulants, such as methylphenidate transdermal system, can cause vasoconstriction and thereby decrease placental perfusion. No fetal and/or neonatal adverse reactions have been reported with the use of therapeutic doses of methylphenidate during pregnancy; however, premature delivery and low birth weight infants have been reported in amphetamine-dependent mothers.
Animal Data
Animal reproduction toxicity studies with transdermal methylphenidate have not been performed. In embryo-fetal development studies conducted in rats and rabbits, methylphenidate was administered orally to pregnant animals during the period of organogenesis, at doses up to 100 and 200 mg/kg/day, respectively. No evidence of morphological development effects was found either of the species; however, increased incidences of fetal skeletal variations were observed in rats at 60 mg/kg or greater and an increase in fetal visceral variations was seen in rabbits at the highest dose. In a previous study, methylphenidate was shown to have malformations (increased incidence of fetal spina bifida) in rabbits when given oral doses of 200 mg/kg/day. When methylphenidate was administered orally to rats throughout pregnancy and lactation at doses of up to 60 mg/kg/day, offspring growth and survival were decreased at maternally toxic doses.
In a study in which oral methylphenidate was given to rats throughout pregnancy and lactation at doses up to 60 mg/kg/day, offspring weights and survival were decreased at 40 mg/kg/day and above; these doses caused some maternal toxicity.
Clinical Considerations
Monitor breastfeeding infants for adverse reactions, such as agitation, insomnia, anorexia, and reduced weight gain.
8.4 Pediatric Use
Methylphenidate transdermal system should not be used in children under six years of age, since safety and efficacy in this age group have not been established. Long-term effects of methylphenidate in children have not been well established.
Long Term Suppression Of Growth
Growth should be monitored during treatment with stimulants, including methylphenidate transdermal system. Children who are not growing or gaining weight as expected may need to have their treatment interrupted [see Warnings and Precautions (5.6)].
Juvenile Animal Toxicity Data
Rats treated with methylphenidate early in the postnatal period through sexual maturation demonstrated a decrease in spontaneous locomotor activity in adulthood. A deficit in acquisition of a specific learning task was observed in females only.
Studies with transdermal methylphenidate have not been performed in juvenile animals. In a study conducted in young rats, methylphenidate was administered orally at doses of up to 100 mg/kg/day for 9 weeks, starting early in the postnatal period (Postnatal Day 7) and continuing through sexual maturity (Postnatal Week 10). When these animals were tested as adults (Postnatal Weeks 13-14), decreased spontaneous locomotor activity was observed in males and females previously treated with 50 mg/kg/day or greater, and a deficit in the acquisition of a specific learning task was seen in females exposed to the highest dose. The no effect level for juvenile neurobehavioral development in rats was 5 mg/kg/day. The clinical significance of the long-term behavioral effects observed in rats is unknown.
8.5 Geriatric Use
Methylphenidate transdermal system has not been studied in patients greater than 65 years of age.
9.1 Controlled Substance
Methylphenidate transdermal system is classified as a Schedule II controlled substance by federal regulation.
9.2 Abuse
See warning containing drug abuse information [see Boxed Warning].
9.3 Dependence
See warning containing drug dependence information [see Boxed Warning].
10.1 Signs And Symptoms
Signs and symptoms of acute methylphenidate overdosage, resulting principally from overstimulation of the CNS and from excessive sympathomimetic effects, may include the following: vomiting, agitation, tremors, hyperreflexia, muscle twitching, convulsions (may be followed by coma), euphoria, confusion, hallucinations, delirium, sweating, flushing, headache, hyperpyrexia, tachycardia, palpitations, cardiac arrhythmias, hypertension, mydriasis, dryness of mucous membranes, and rhabdomyolysis.
10.2 Recommended Treatment
Remove all transdermal systems immediately and cleanse the area(s) to remove any remaining adhesive. The continuing absorption of methylphenidate from the skin, even after removal of the transdermal system, should be considered when treating patients with overdose. Treatment consists of appropriate supportive measures. The patient must be protected against self-injury and against external stimuli that would aggravate overstimulation already present. Intensive care must be provided to maintain adequate circulation and respiratory exchange; external cooling procedures may be required for hyperpyrexia.
Efficacy of peritoneal dialysis or extracorporeal hemodialysis for methylphenidate transdermal system overdosage has not been established.
10.3 Poison Control Center
As with the management of all overdosages, the possibility of multiple drug ingestion should be considered. The physician may wish to consider contacting a poison control center for up-to-date information on the management of overdosage with methylphenidate.
11 Description
Methylphenidate transdermal system is an adhesive-based matrix transdermal system containing methylphenidate that is applied to intact skin. The chemical name for methylphenidate is α-phenyl-2-piperidineacetic acid methyl ester. It is a white to off-white powder and is soluble in alcohol, ethyl acetate, and ether. Methylphenidate is practically insoluble in water and petrol ether. Its molecular weight is 233.31. Its molecular formula is C14H19NO2. The structural formula of methylphenidate is:
11.1 Transdermal System Components
Methylphenidate transdermal system contains methylphenidate in a polyisobutylene adhesive. The composition per unit area of all dosage strengths is identical, and the total dose delivered is dependent on the transdermal system size and wear time.
The transdermal system consists of four layers, as seen in the figure below (cross-section of the transdermal system)
Proceeding from the outer surface toward the surface adhering to the skin, the layers are (1) a polyester/ethylene vinyl acetate laminate film backing and white ink which contains acrylic polymers, polyethylene wax, polytetrafluoroethylene, polyvinylpyrrolidone, sodium dioctyl sulfosuccinate and titanium dioxide, (2) a solid matrix drug reservoir of methylphenidate, polyisobutylene adhesive, mineral oil and hydrophobic colloidal silica, (3) a skin contact adhesive formulation of polyisobutylene adhesive, mineral oil and hydrophobic colloidal silica, and (4) a fluoropolymer-coated polyester protective liner, which is attached to the adhesive surface and must be removed before the transdermal system can be used.
The active component of the transdermal system is methylphenidate. The remaining components are pharmacologically inactive.
Methylphenidate transdermal systems are packaged with an additional piece of protective film above the system within each pouch. This piece of protective film is removed and discarded at the time of use.
12.1 Mechanism Of Action
Methylphenidate is a central nervous system (CNS) stimulant. Its mode of therapeutic action in Attention Deficit Hyperactivity Disorder (ADHD) is not known.
12.2 Pharmacodynamics
Methylphenidate is a racemic mixture comprised of the d- and l-enantiomers. The d-enantiomer is more pharmacologically active than the l-enantiomer. Methylphenidate blocks the reuptake of norepinephrine and dopamine into the presynaptic neuron and increases the release of these monoamines into the extraneuronal space.
12.3 Pharmacokinetics
The pharmacokinetics of methylphenidate transdermal system when applied to the hip for 9 hours have been studied in ADHD patients 6 to 17 years old.
Absorption
The amount of methylphenidate absorbed systemically is a function of both wear time and transdermal system size. In patients with ADHD, peak plasma levels of methylphenidate are reached at about 10 hours after single application and 8 hours after repeat transdermal system applications (12.5 cm² to 37.5 cm²) when worn up to 9 hours.
On single dosing of children or adolescents with methylphenidate transdermal system, there was a delay of, on average, 2 hours before d-methylphenidate was detectable in the circulation. On repeat dosing, low concentrations (1.2-3.0 ng/mL in children and 0.5-1.7 ng/mL in adolescents, on average across the dose range) were observed earlier in the profile, due to carry-over effect. Following the application of methylphenidate transdermal system once daily with a 9-hour wear time, the mean pharmacokinetic parameters of d-methylphenidate in children and adolescents with ADHD after 4 weeks of therapy are summarized in Table 3.
Children | ||||
Parameter | Methylphenidate Dose maintained fixed for 28 days; 12.5 cm² (N = 12) | Methylphenidate Dose escalated at 7 day intervals from 12.5 cm² through 18.75 cm² and 25 cm² to 37.5 cm²; 37.5 cm² (N = 10) | Oral ER-MPH Dose escalated at 7 day intervals from 18 mg through 27 mg and 36 mg to 54 mg; 18 mg | Oral ER-MPH |
Cssmax (ng/mL) | 15.7 ± 9.39 | 42.9 ± 22.4 | 8.37 ± 4.14 | 26.1 ± 11.2 |
Cssmin (ng/mL) | 1.04 ± 1.17 | 1.96 ± 1.73 | 0.708 ± 1.08 | 1.19 ± 1.54 |
AUCss (ng∙hr/mL) | 163 ± 101 | 447 ± 230 | 97.7 ± 67.0 | 317 ± 160 |
tlag (h) Median (minimum – maximum); tlag = Last Sampling Time Prior to Time of First Quantifiable Plasma Concentration | 0 (0 - 2.0) | 0 (0 - 1.0) | 0 | 0 |
Adolescents | ||||
Cssmax (ng/mL) | 8.32 ± 4.60 | 16.5 ± 6.94 | 5.23 ± 1.72 | 18.0 ± 6.97 |
Cssmin (ng/mL) | 0.544 ± 0.383 | 1.02 ± 0.629 | 0.360 ± 0.478 | 1.50 ± 0.937 |
AUCss (ng∙hr/mL) | 85.7 ± 50.0 | 167 ± 66.0 | 59.7 ± 19.1 | 216 ± 80.8 |
tlag (h) | 0 (0 - 2.0) | 0 (0 - 2.0) | 0 | 0 |
Following administration of methylphenidate transdermal system 12.5 cm2 to pediatric and adolescent ADHD patients daily for 7 days, there were 13% and 14% increases, respectively, in steady state area under the plasma concentration-time curve (AUCss) relative to that anticipated on the basis of single dose pharmacokinetics (AUC0-∞); after 28 days administration, these increments increased to 64% and 76%, respectively. Cmax increased by nearly 69% and 100% within 4 weeks of daily administration of the starting dose in children and adolescents, respectively.
The observed exposures with methylphenidate transdermal system could not be explained by drug accumulation predicted from observed single dose pharmacokinetics and there was no evidence that clearance or rate of elimination changed between single and repeat dosing. Neither were they explainable by differences in dosing patterns between treatments, age, race, or gender. This suggests that transdermal absorption of methylphenidate may increase with repeat dosing with methylphenidate transdermal system; on average, steady-state is likely to have been achieved by approximately 14 days of dosing.
In the single- and multiple dose study described above, exposure to l-methylphenidate was 46% of the exposure to d-methylphenidate in children and 40% in adolescents. l-methylphenidate is less pharmacologically active than d-methylphenidate [see Pharmacodynamics (12.2)].
In a phase 2 PK/PD study in children aged 6-12 years, 2/3 of patients had 2-hour d-MPH concentrations < 5 ng/mL on chronic dosing, and at 3 hours 40% of patients had d-MPH concentrations < 5 ng/mL [see Clinical Studies (14)].
When methylphenidate transdermal system is applied to inflamed skin both the rate and extent of absorption are increased as compared with intact skin. When applied to inflamed skin, lag time is no greater than 1 hour, Tmax is 4 hours, and both Cmax and AUC are approximately 3-fold higher.
When heat is applied to methylphenidate transdermal system after transdermal system application, both the rate and the extent of absorption are significantly increased. Median Tlag occurs 1 hour earlier, Tmax occurs 0.5 hours earlier, and median Cmax and AUC are 2-fold and 2.5-fold higher, respectively.
Application sites other than the hip can have different absorption characteristics and have not been adequately studied in safety or efficacy studies.
Dose Proportionality
Following a single 9-hour application of methylphenidate transdermal system doses of 10 mg/9 hours to 30 mg/9 hours transdermal systems to 34 children with ADHD, Cmax and AUC0-t of d-methylphenidate were proportional to the transdermal system dose. Mean plasma concentration-time plots are shown in Figure 1. Cmax of l-methylphenidate was also proportional to the transdermal system dose. AUC0-t of l‑methylphenidate was only slightly greater than proportional to transdermal system dose.
FIGURE 1
Mean Concentration-time Profiles for d-Methylphenidate in all Patients (N = 34) Following Administration of Single Applications (9-Hour Wear Time) of d,l-Methylphenidate Using Methylphenidate Transdermal System 10 mg (□), 20 mg (◊) and 30 mg (Δ) per 9-Hour Transdermal Systems
Distribution
Upon removal of methylphenidate transdermal system, methylphenidate plasma concentrations in children with ADHD decline in a biexponential manner. This may be due to continued distribution of MPH from the skin after transdermal system removal.
Metabolism And Excretion
Methylphenidate is metabolized primarily by de-esterification to alpha-phenyl-piperidine acetic acid (ritalinic acid), which has little or no pharmacologic activity.
Transdermal administration of methylphenidate exhibits much less first pass effect than oral administration. Consequently, a much lower dose of methylphenidate transdermal system on a mg/kg basis compared to oral dosages may still produce higher exposures of d-MPH with transdermal administration compared to oral administration. In addition, very little, if any, l-methylphenidate is systemically available after oral administration due to first pass metabolism, whereas after transdermal administration of racemic methylphenidate exposure to l-methylphenidate is nearly as high as to d-methylphenidate.
The mean elimination t1/2 from plasma of d-methylphenidate after removal of methylphenidate transdermal system in children aged 6 to 12 years and adolescents aged 13-17 years was approximately 4 to 5 hours. The t1/2 of l-methylphenidate was shorter than for d-methylphenidate and ranged from 1.4 to 2.9 hours, on average.
The Cmax and AUC of d-methylphenidate were approximately 50% lower in adolescents, compared to children, following either a 1-day or 7-day administration of methylphenidate transdermal system (10 mg/9 hr). Multiple-dose administration of methylphenidate transdermal system did not result in significant accumulation of methylphenidate; following 7 days of methylphenidate transdermal system administration (10 mg/9 hr) in children and adolescents, the accumulation index of methylphenidate was 1.1, based on the mean steady state area under the plasma concentration-time curve (AUCss) relative to that anticipated on the basis of single dose pharmacokinetics (AUC0-∞).
Food Effects
The pharmacokinetics or the pharmacodynamic food effect performance after application of methylphenidate transdermal system has not been studied, but because of the transdermal route of administration, no food effect is expected.
Gender
The pharmacokinetics of methylphenidate after single and repeated doses of methylphenidate transdermal system were similar between boys and girls with ADHD, after allowance for differences in body weight.
Race
The influence of race on the pharmacokinetics of methylphenidate after administration of methylphenidate transdermal system has not been defined.
Age
The pharmacokinetics of methylphenidate after administration of methylphenidate transdermal system have not been studied in children less than 6 years of age.
Renal Impairment
There is no experience with the use of methylphenidate transdermal system in patients with renal insufficiency.
Hepatic Impairment
There is no experience with the use of methylphenidate transdermal system in patients with hepatic insufficiency.
Carcinogenesis
Carcinogenicity studies of transdermal methylphenidate have not been performed. In a lifetime carcinogenicity study of oral methylphenidate carried out in B6C3F1 mice, methylphenidate caused an increase in hepatocellular adenomas and, in males only, an increase in hepatoblastomas, at a daily dose of approximately 60 mg/kg/day. Hepatoblastoma is a relatively rare rodent malignant tumor type. There was no increase in total malignant hepatic tumors. The mouse strain used is sensitive to the development of hepatic tumors and the significance of these results to humans is unknown.
Orally administered methylphenidate did not cause any increases in tumors in a lifetime carcinogenicity study carried out in F344 rats; the highest dose used was approximately 45 mg/kg/day.
In a 24-week oral carcinogenicity study in the transgenic mouse strain p53+/-, which is sensitive to genotoxic carcinogens, there was no evidence of carcinogenicity. In this study, male and female mice were fed diets containing the same concentration of methylphenidate as in the lifetime carcinogenicity study; the high-dose groups were exposed to 60 to 74 mg/kg/day of methylphenidate.
Mutagenesis
Methylphenidate was not mutagenic in the in vitro Ames reverse mutation assay or in the in vitro mouse lymphoma cell forward mutation assay. Sister chromatid exchanges and chromosome aberrations were increased, indicative of a weak clastogenic response, in an in vitro assay in cultured Chinese hamster ovary cells. Methylphenidate was negative in vivo in males and females in the mouse bone marrow micronucleus assay.
Impairment Of Fertility
Methylphenidate did not impair fertility in male or female mice that were fed diets containing the drug in an 18‑week continuous breeding study. The study was conducted at doses up to 160 mg/kg/day.
14 Clinical Studies
Methylphenidate transdermal system was demonstrated to be effective in the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in two (2) randomized double-blind, placebo-controlled studies in children aged 6 to 12 years and one (1) randomized, double-blind, placebo-controlled study in adolescents aged 13 to 17 years who met Diagnostic and Statistical Manual (DSM-IV-TR®) criteria for ADHD. The transdermal system wear time was 9 hours in all three (3) studies.
In Study 1, conducted in a classroom setting, symptoms of ADHD were evaluated by school teachers and observers using the Deportment Subscale from the Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) rating scale which assesses behavior symptoms in the classroom setting. Methylphenidate transdermal system was applied for 9 hours before removal. There was a 5-week open-label methylphenidate transdermal system dose optimization phase using dosages of 10 mg/9 hours, 15 mg/9 hours, 20 mg/9 hours, and 30 mg/9 hours, followed by a 2-week randomized, double-blind, placebo-controlled crossover treatment phase using the optimal transdermal system dose for each patient or placebo. The mean differences between methylphenidate transdermal system and placebo in change from baseline in SKAMP Deportment Scores were statistically significant in favor of methylphenidate transdermal system beginning at 2 hours and remained statistically significant at all subsequent measured time points through 12 hours after application of the methylphenidate transdermal system.
In Study 2, conducted in the outpatient setting, methylphenidate transdermal system or placebo was blindly administered in a flexible-dose design using doses of 10 mg/9 hours, 15 mg/9 hours, 20 mg/9 hours, and 30 mg/9 hours to achieve an optimal regimen over 5 weeks, followed by a 2-week maintenance period using the optimal transdermal system dose for each patient. Symptoms of ADHD were evaluated by the ADHD-Rating Scale (RS)-IV. Methylphenidate transdermal system was statistically significantly superior to placebo as measured by the mean change from baseline for the ADHD-RS-IV total score. Although this study was not designed specifically to evaluate dose response, in general there did not appear to be any additional effectiveness accomplished by increasing the transdermal system dose from 20 mg/9 hours to 30 mg/9 hours.
In Study 3, conducted in the outpatient setting, methylphenidate transdermal system or placebo was blindly administered in a flexible-dose design using doses of 10 mg/9 hours, 15 mg/9 hours, 20 mg/9 hours, and 30 mg/9 hours during a 5-week dose-optimization phase, followed by a 2-week maintenance period using the optimal transdermal system dose for each patient. Symptoms of ADHD were evaluated using the ADHD-Rating Scale (RS)-IV. Methylphenidate transdermal system was statistically significantly superior to placebo as measured by the mean change from baseline in the ADHD-RS-IV total score.
15 References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association 1994.
16 How Supplied/Storage And Handling
Methylphenidate Transdermal System is supplied in a carton containing 30 individually pouched transdermal systems. See the chart below for information regarding available strengths.
Each dosage form is a translucent rectangular transdermal system with rounded corners consisting of a matte backing film randomly printed with “Methylphenidate Transdermal System” and the “mg/hr” strength in white ink, an adhesive layer and a clear to slightly hazy oversized release liner that is slit. Each transdermal system is overlaid with an additional clear to slightly hazy oversized release liner and is contained in a square, flat pouch that is imprinted with lot number and expiration date.
Nominal Dose Delivered (mg) Over 9 Hours | Dosage Rate Nominal in vivo delivery rate per hour in children and adolescents when applied to the hip, based on a 9-hour wear period. (mg/hr) | Transdermal System Size (cm²) | Methylphenidate Content per Transdermal System Methylphenidate content in each transdermal system. (mg) | Transdermal Systems Per Carton | NDC Number |
10 | 1.1 | 9.6 | 10.4 | 30 | 0378-8260-93 |
15 | 1.6 | 14.4 | 15.6 | 30 | 0378-8261-93 |
20 | 2.2 | 19.2 | 20.7 | 30 | 0378-8262-93 |
30 | 3.3 | 28.8 | 31.1 | 30 | 0378-8263-93 |
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature]. Do not store transdermal systems unpouched. Do not store transdermal systems in refrigerators or freezers.
Apply the transdermal system immediately upon removal from the individual protective pouch. For transdermal use only.
PHARMACIST: Dispense a Medication Guide with each prescription.
17 Patient Counseling Information
Advise patients to read the FDA-approved patient labeling (Medication Guide).
Priapism: Advise patients, caregivers, and family members of the possibility of painful or prolonged penile erections (priapism). Instruct the patient to seek immediate medical attention in the event of priapism [see Warnings and Precautions (5.4)].
Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud’s phenomenon]:
• Instruct patients beginning treatment with methylphenidate transdermal system about the risk of peripheral vasculopathy, including Raynaud’s phenomenon, and its associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red• Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.• Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while using methylphenidate transdermal system• Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
Chemical Leukoderma: Advise patients of the possibility of a persistent loss of skin pigmentation at, around and distant from the application site. Advise patients to immediately inform their healthcare provider if changes in skin pigmentation occur [see Warnings and Precautions (5.7)].
Parents and patients should be informed to apply methylphenidate transdermal system to a clean, dry site on the hip, which is not oily, damaged, or irritated. The site of application must be alternated daily. The transdermal system should not be applied to the waistline, or where tight clothing may rub it.
If patients or caregivers experience difficulty separating the transdermal system from the release liner or observe tearing and/or other damage to the transdermal system during removal from the liner, the transdermal system should be discarded according to the directions provided in this label, and a new transdermal system should be applied [see Dosage and Administration (2.3)]. Patients or caregivers should inspect the release liner to ensure that no adhesive containing medication has transferred to the liner. If adhesive transfer has occurred, the transdermal system should be discarded.
Methylphenidate transdermal system should be applied 2 hours before the desired effect. Methylphenidate transdermal system should be removed approximately 9 hours after it is applied, although the effects from the transdermal system will last for several more hours. Methylphenidate transdermal system may be removed earlier than 9 hours if a shorter duration of effect is desired or late day side effects appear.
The parent or caregiver should be encouraged to use the administration chart included with each carton of methylphenidate transdermal system to monitor application and removal time, and method of disposal. The Medication Guide included at the end of this insert also includes a timetable to calculate when to remove methylphenidate transdermal system, based on the 9 hour application time.
Patients or caregivers should avoid touching the adhesive side of the transdermal system during application, in order to avoid absorption of methylphenidate. If they do touch the adhesive side of the transdermal system, they should immediately wash their hands after application.
In the event that a transdermal system does not fully adhere to the skin upon application, or is partially or fully detached during wear time, the transdermal system should be discarded according to the directions provided in this label, and a new transdermal system should be applied [see Dosage and Administration (2.3)]. If a transdermal system is replaced, the total recommended wear time for that day should remain 9 hours, regardless of the number of transdermal systems used.
Transdermal systems should not be applied or re-applied with dressings, tape, or other common adhesives.
Exposure to water during bathing, swimming, or showering can affect transdermal system adherence.
Do not cut transdermal systems. Only intact transdermal systems should be applied.
If there is an unacceptable duration of appetite loss or insomnia in the evening, taking the transdermal system off earlier may be attempted before decreasing the transdermal system dose.
Skin redness or itching is common with methylphenidate transdermal system and small bumps on the skin may also occur in some patients. If any swelling or blistering occurs the transdermal system should not be worn and the patient should be seen by the prescriber. Patients or caregivers should not apply hydrocortisone or other solutions, creams, ointments, or emollients immediately prior to transdermal system application, since the effect on transdermal system adhesion and methylphenidate absorption has not been established. The potential adverse effects of topical corticosteroid use during treatment with methylphenidate transdermal system are unknown.
Stimulants may impair the ability of the patient to operate potentially hazardous machinery or vehicles. Patients should be cautioned accordingly until they are reasonably certain that methylphenidate transdermal system does not adversely affect their ability to engage in such activities.
Transdermal systems should be stored at 20° to 25°C (68° to 77°F) [see How Supplied/Storage and Handling (16)]. Patients or caregivers should be advised not to store methylphenidate transdermal system in the refrigerator or freezer.
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with methylphenidate transdermal system and should counsel them in its appropriate use. A patient Medication Guide is available for methylphenidate transdermal system. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is printed at the end of this document.
Pregnancy Registry: Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ADHD medications, including methylphenidate transdermal system, during pregnancy [see Use in Specific Populations (8.1)].
For more information, call Mylan at 1-877-446-3679 (1-877-4-INFO-RX).
Medication Guide
Methylphenidate Transdermal System CII
(meth'' il fen' i date)
Only Use Methylphenidate Transdermal System on Your Skin |
Important: |
What is the most important information I should know about methylphenidate transdermal system?
Methylphenidate transdermal system contains methylphenidate which is a central nervous system (brain) stimulant medicine. Serious side effects have been reported with methylphenidate transdermal system or other stimulant medicines, including:
1. Heart problems, including:
• sudden death in people who have heart problems or heart defects• stroke and heart attack in adults• increased blood pressure and heart rate• chest pain• shortness of breath• fainting• new or worse aggressive behavior, hostility, anger, or irritability• new or worse bipolar illness or mania (an extreme increase in activity or talking)• new or worse psychosis (hearing or seeing things that are not real, being suspicious or distrustful, believing things that are not true)• other unusual or extreme changes in behavior or mood• Tell your doctor if you have or your child has numbness, pain, skin color change, or sensitivity to temperature in your fingers or toes.• Call your doctor right away if you have or your child has any signs of unexplained wounds appearing on fingers or toes while taking methylphenidate transdermal system• are very anxious, tense, or agitated• have glaucoma• have tics (involuntary repeated movements or sounds that cannot be controlled)• have Tourette’s Syndrome or a family history of this syndrome• are taking or have taken a monoamine oxidase inhibitor (MAOI) medicine within the past 2 weeks. Do not take a MAOI medicine for at least 2 weeks before using methylphenidate transdermal system. Ask your doctor or pharmacist if you are not sure if any of your medicines are MAOIs.• are allergic to methylphenidate or any other ingredients in methylphenidate transdermal system. See “What are the ingredients in methylphenidate transdermal system?” for a complete list of ingredients.• have heart problems, heart defects, high blood pressure• have mental problems including psychosis, mania, bipolar illness, or depression• have seizures or have had an abnormal brain wave test (EEG)• have circulation problems in fingers or toes• have skin problems such as eczema or psoriasis, or have skin reactions to soaps, lotions, make-up, or adhesives (glues)• are pregnant or plan to become pregnant. It is not known if methylphenidate transdermal system will harm your unborn baby.o There is a pregnancy registry for females who are exposed to ADHD medications, including methylphenidate transdermal system during pregnancy. The purpose of the registry is to collect information about the health of females exposed to methylphenidate transdermal system and their baby. If you or your child becomes pregnant during treatment with methylphenidate transdermal system, talk to your healthcare provider about registering with the National Pregnancy Registry of ADHD Medications at 1-866-961-2388 or visit online at https://womensmentalhealth.org/adhd-medications/.
• are breast feeding or plan to breast feed. Methylphenidate passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with methylphenidate transdermal system.• a history of vitiligo and/or a family history of vitiligo• a monoamine oxidase inhibitor (MAOI) medicine See “Who should not use methylphenidate transdermal system?”• medicines to treat depression• medicines to treat seizures• a blood pressure medicine• a blood thinner medicine• cold or allergy medicines that contain decongestants• Read the Patient Instructions for Use at the end of this Medication Guide that comes with your methylphenidate transdermal system for information about the right way to use methylphenidate transdermal system.• Use methylphenidate transdermal system exactly as your doctor tells you to.• Your doctor may change your dose if needed.• Apply methylphenidate transdermal system to your hip 2 hours before an effect is needed.• Do not wear methylphenidate transdermal system longer than 9 hours a day.• Apply methylphenidate transdermal system to a different hip each day.• Do not cut methylphenidate transdermal systems.• Parents or caregivers should apply and remove methylphenidate transdermal system for their child if the child is not responsible enough to do so.• Your doctor may stop methylphenidate transdermal system treatment to check your ADHD symptoms.• Your doctor may do certain blood tests and check your heart and blood pressure while you use methylphenidate transdermal system.• If you forget to apply a transdermal system in the morning, you may apply the transdermal system later in the day. You should remove your transdermal system at the usual time of day to lower the chance of side effects later in the day.• If you have loss of appetite or trouble sleeping in the evening, ask your doctor if you can take the transdermal system off earlier in the day.• Contact with water while bathing, swimming, or showering can make the transdermal system not stick well or make it fall off. If your transdermal system falls off, do not touch the sticky side of the transdermal system with your fingers. You may apply a new transdermal system to a different area on the same hip. If you have to replace a transdermal system that has fallen off, the total wear time for the first and second transdermal system should not be more than a total of 9 hours in 1 day. Do not reapply the same transdermal system that fell off.• Do not wear your methylphenidate transdermal system longer than 9 hours.• If you accidentally apply or wear more than 1 transdermal system at a time, you have used too many methylphenidate transdermal systems. Remove all transdermal systems, wash the application sites right away and call your doctor.o Call your Poison Control Center at 1-800-222-1222 -or go to the nearest hospital emergency room right away if you have:
o vomitingo agitationo shakingo confusion or mental changeso see things that are not there (hallucinations)o sweatingo redness in your faceo headacheo heartbeat changes• Do not put any medicine, cream, or lotion on your hip before you apply the methylphenidate transdermal system. Medicines, creams or lotions may affect how the transdermal system sticks to your skin and how the medicine is absorbed from the transdermal system.• Do not use bandages, tape, or other household adhesives (glue) to hold the transdermal system onto your skin.• Do not use hair dryers, heating pads, electric blankets, heated water beds or other heat sources while wearing a methylphenidate transdermal system. Too much medicine can pass into your body and cause serious side effects.• Do not drive, operate heavy machinery or do other dangerous activities until you know how methylphenidate transdermal system affects you.• See “What is the most important information I should know about methylphenidate transdermal system?”• Seizures. This usually happens in people with a history of seizures.• Painful and prolonged erections (priapism) have occurred with methylphenidate. If you or your child develops priapism, seek medical help right away. Because of the potential for lasting damage, priapism should be evaluated by a doctor immediately.• Slowing of growth (weight and height). You should have your height and weight checked while using methylphenidate transdermal system.• Loss of skin color (chemical leukoderma). Methylphenidate transdermal system may cause a persistent loss of skin-color where the transdermal system is applied or around the transdermal system application site. Loss of skin-color, in some cases, has been reported at locations on the skin far from any application site. The loss of your skin-color may be permanent even after removing the transdermal system or stopping use of methylphenidate transdermal system. Call your doctor immediately if you have changes in your skin-color.• Allergic skin rash. Stop using methylphenidate transdermal system and see your doctor right away if you have swelling or blisters at or around the application site. You may have a skin allergy to methylphenidate transdermal system. People who have skin allergies to methylphenidate transdermal system may develop an allergy to all medicines that contain methylphenidate, even those methylphenidate medicines that are taken by mouth.• Eyesight changes or blurred vision• skin problems where you apply• poor appetite• nausea• vomiting• stomach pain• weight loss• tics• trouble sleeping• mood swings• dizziness• Store methylphenidate transdermal system at room temperature between 20° to 25°C (68° to 77°F).• Do not store methylphenidate transdermal system in the refrigerator or freezer.• Keep methylphenidate transdermal systems in their unopened pouches until you are ready to use them.• when you apply the transdermal system to the skin on your hip each morning• when you remove the transdermal system• how and where you threw the methylphenidate transdermal system away• Each day, when a new methylphenidate transdermal system is applied to your hip, write down the date and time that you applied the transdermal system.• Use the methylphenidate transdermal system schedule below so you can decide when to remove the transdermal system. For example, if the transdermal system is applied to the skin at 6:00 a.m., remove the transdermal system at 3:00 p.m. on the same day. After you remove and throw away the transdermal system, write down the time you removed the transdermal system and how and where you threw it away.• If the transdermal system you placed on your child is missing, ask your child:o when the transdermal system came offo how the transdermal system came offo where the transdermal system is
• Apply the transdermal system to your hip area. Do not put the transdermal system near your waist. Clothing and movement may make your transdermal system rub off (See Figure A).• Use your other hip when you apply a new transdermal system the next morning. Make sure there is no redness, small bumps or itching at the site where the transdermal system is going to be applied.• Is clean (freshly washed), dry, and cool• Does not have any powder, oil, or lotion• Does not have any cuts and irritation (rashes, inflammation, redness, or other skin problems).• Each transdermal system is sealed in its own protective pouch.• Carefully cut the protective pouch open with scissors, being careful not to cut the transdermal system.• Remove the transdermal system from the protective pouch and discard additional piece of protective film above the transdermal system.• Look at the transdermal system to make sure it is not damaged. The transdermal system should separate easily from the protective liner. Throw away the transdermal system if the protective liner is hard to remove.• Protective liner: The protective liner is the layer that you remove before you put the transdermal system on (See Figure C).• Adhesive layers with medicine: The adhesive layers with medicine consist of an adhesive reservoir layer that contains drug and an adhesive layer that sticks to your skin (See Figure C).• Outside backing: The outside backing is the layer that you see after you put the transdermal system on your skin. The words “Methylphenidate Transdermal System” are printed on this layer (See Figure D).• Apply the transdermal system right away after you remove the transdermal system from protective pouch.• Hold the transdermal system with the hard protective liner facing you. The words “Methylphenidate Transdermal System” will appear backwards.• Gently bend the transdermal system along the faint line and slowly peel one side of the liner, which covers the sticky surface of the transdermal system (See Figure E).• Avoid touching the sticky side of the transdermal system with your fingers.• If you accidentally touch the sticky side of the transdermal system, apply the transdermal system, then wash your hands right away so that the medicine does not go into the skin on your hands.• Using the other side of the protective liner as a handle, apply the sticky side of the transdermal system to the selected area of the child’s hip (See Figure F).• Press the sticky side of the transdermal system firmly into place and smooth it down.• While you are still holding the sticky side down, gently fold back the other side of the transdermal system.• Hold an edge of the remaining protective liner and slowly peel it off (See Figure G).• After the protective liner is removed, there should not be any adhesive (glue) sticking to the liner.• Press the entire transdermal system firmly into place with the palm of your hand over the transdermal system for about 30 seconds (See Figure H).• Make sure that the transdermal system firmly sticks to your skin.• Gently rub the edges of the transdermal system with your fingers to make sure the transdermal system sticks to your skin.• Wash your hands after you apply your transdermal system.• Write the time you applied your transdermal system on the dosing chart on the carton. Use the dosing schedule so you know what time you should remove your transdermal system.• When you remove the transdermal system, peel it off slowly. If the transdermal system is too sticky on your skin and you need something to help you remove it:o Gently apply an oil-based product (petroleum jelly, olive oil, or mineral oil) to the transdermal system edges. Gently spread the oil underneath the transdermal system edges.o Apply an oil-based product or lotion to your skin if any adhesive (glue) remains after you remove your transdermal system. This will gently loosen and remove any adhesive that is left over.o If you still cannot easily remove the transdermal system, ask your doctor or pharmacist about what to do for this problem.
• Fold the used methylphenidate transdermal system in half and press it together firmly so that the sticky side sticks to itself. Flush the used transdermal system down the toilet or put the transdermal system in a container with a lid right away.• Do not flush the protective pouches or the protective liners down the toilet. These items should be thrown away in a container with a lid.• Wash your hands after you handle the transdermal system.• After you remove the transdermal system and throw the transdermal system away, write down the time on the dosing chart.• Safely throw away any unused methylphenidate transdermal systems that are left over from the prescription as soon as they are no longer needed.
To safely throw away the transdermal systems:o Remove the leftover transdermal systems from their protective pouches and remove the protective liners.o Either fold the transdermal systems in half with the sticky sides together, and flush the transdermal systems down the toilet, oro Throw the transdermal systems away in a container with a lid.
Your doctor should check you carefully for blood pressure and heart problems before you start and while you are using methylphenidate transdermal system.
Remove the methylphenidate transdermal system and call your doctor right away if you have any signs of heart problems such as:
2. Mental (psychiatric) problems, including:
Tell your doctor right away if you have any new or worsening mental problems while using methylphenidate transdermal system.
3. Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud’s phenomenon]: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red
What is methylphenidate transdermal system?
Methylphenidate transdermal system is a prescription medication used to treat Attention Deficit Hyperactivity Disorder (ADHD) in people 6 to 17 years old. Methylphenidate transdermal system is a central nervous system (brain) stimulant medicine. Methylphenidate transdermal system may help you have better attention and less impulsive and hyperactive behavior. Methylphenidate transdermal system is a transdermal system that you apply to your skin on your hip. Methylphenidate transdermal system is used as part of a total treatment program for ADHD that may also include counseling or other treatments.
It is not known if methylphenidate transdermal system is safe and effective in children younger than 6 years.
Who should not use methylphenidate transdermal system?
Do not use methylphenidate transdermal system if you:
Talk to your healthcare provider before taking this medicine if you have any of these conditions.
What should I tell my doctor before using methylphenidate transdermal system?
Before you start using methylphenidate transdermal system, tell your doctor if you:
Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Methylphenidate transdermal system and certain other medicines may affect each other, causing serious side effects.
Especially tell your doctor if you take:
Know the medicines that you take. Keep a list of them to show your doctor and pharmacist. Do not start any new medicine while using methylphenidate transdermal system without talking to your doctor first.
How should I use methylphenidate transdermal system?
What should I avoid while using methylphenidate transdermal system?
What are possible side effects of methylphenidate transdermal system?
Methylphenidate transdermal system may cause serious side effects, including:
The most common side effects of methylphenidate transdermal system include:
Tell your doctor if you have any side effect that bothers you or does not go away.
These are not all the possible side effects of methylphenidate transdermal system. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store methylphenidate transdermal system?
Keep methylphenidate transdermal system and all medicines out of the reach of children.
General information about the safe and effective use of methylphenidate transdermal system.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use methylphenidate transdermal system for a condition for which it was not prescribed. Do not give methylphenidate transdermal system to other people, even if they have the same symptoms that you have. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about methylphenidate transdermal system. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about methylphenidate transdermal system that is written for health professionals.
For more information, call Mylan at 1-877-446-3679 (1-877-4-INFO-RX).
What are the ingredients in methylphenidate transdermal system?
Active ingredient: methylphenidate
Inactive ingredients: fluoropolymer-coated polyester release liner, hydrophobic colloidal silica, mineral oil, polyester/ethylene vinyl acetate laminate backing film and polyisobutylene adhesive. The white ink contains acrylic polymers, polyethylene wax, polytetrafluoroethylene, polyvinylpyrrolidone, sodium dioctyl sulfosuccinate and titanium dioxide.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Instructions for Use
Methylphenidate Transdermal System CII
(meth'' il fen' i date)
1. Methylphenidate Transdermal System Dosing Chart
Each carton of methylphenidate transdermal systems contains a methylphenidate transdermal system Dosing Chart to help you keep track of your transdermal system including:
To use the methylphenidate transdermal system Dosing Chart, follow these instructions:
Methylphenidate Transdermal System Schedule for 9 Hour Dosing
If you put the transdermal system on at: | On the same day, remove the transdermal system at: |
5:00 a.m. | 2:00 p.m. |
6:00 a.m. | 3:00 p.m. |
7:00 a.m. | 4:00 p.m. |
8:00 a.m. | 5:00 p.m. |
9:00 a.m. | 6:00 p.m. |
10:00 a.m. | 7:00 p.m. |
11:00 a.m. | 8:00 p.m. |
12:00 p.m. | 9:00 p.m. |
2. Where to apply methylphenidate transdermal system
Figure A
3. Before you apply methylphenidate transdermal system
Make sure your skin:
4. How to apply methylphenidate transdermal system
Figure B
The methylphenidate transdermal system has 4 layers. The 4 layers are pictured below. The pictures show both sides of the transdermal system:
Figure C Figure D
Layers:
Figure E
Figure F
Figure G
Figure H
5. How to remove and throw away methylphenidate transdermal system
This Medication Guide and Instructions for Use has been approved by the U.S. Food and Drug Administration.
The brands listed are trademarks of their respective owners.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Revised: 7/2021
MPN:R4
Principal Display Panel – 1.1 Mg/Hr
Contains: 30 Systems
NDC 0378-8260-93 Rx only
Methylphenidate
Transdermal System CII
1.1 mg/hr
Delivers 10 mg over 9 hours (1.1 mg/hr)
Transdermal system should be worn for approximately 9 hours.
Each transdermal system contains 10.4 mg of methylphenidate.
Active ingredient release is limited; please see recommended dosing in
patient instructions.
For transdermal use only (applied only to skin).
Important: Keep out of the reach of children.
It is important that this product be disposed of properly.
See patient instructions for disposal information.
Pharmacist: Dispense the accompanying
Medication Guide to each patient.
Inactive components: fluoropolymer-coated polyester release liner,
hydrophobic colloidal silica, mineral oil, polyester/ethylene vinyl acetate
laminate backing film and polyisobutylene adhesive. The white ink contains
acrylic polymers, polyethylene wax, polytetrafluoroethylene,
polyvinylpyrrolidone, sodium dioctyl sulfosuccinate and titanium dioxide.
Keep all transdermal systems within provided containers and dispense one
transdermal system daily.
Apply immediately upon removal from pouch.
Do not store unpouched. Do not store in refrigerators or freezers.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room
Temperature.]
Dosage and Administration: See package insert.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
1-877-446-3679 (1-877-4-INFO-RX)
Mylan.com
M8260:93:30C:R3
Date of Transdermal System Application MM/DD/YYYY | Time Applied | Time Removed | Application Site | Disposal Method |
_AM _PM | _AM _PM | _Right _Left | _Fold & flush _Fold & trash | |
_AM _PM | _AM _PM | _Right _Left | _Fold & flush _Fold & trash |
Principal Display Panel – 1.6 Mg/Hour
Contains: 30 Systems
NDC 0378-8261-93 Rx only
Methylphenidate
Transdermal System CII
1.6 mg/hr
Delivers 15 mg over 9 hours (1.6 mg/hr)
Transdermal system should be worn for approximately 9 hours.
Each transdermal system contains 15.6 mg of methylphenidate.
Active ingredient release is limited; please see recommended dosing in
patient instructions.
For transdermal use only (applied only to skin).
Important: Keep out of the reach of children.
It is important that this product be disposed of properly.
See patient instructions for disposal information.
Pharmacist: Dispense the accompanying
Medication Guide to each patient.
Inactive components: fluoropolymer-coated polyester release liner,
hydrophobic colloidal silica, mineral oil, polyester/ethylene vinyl acetate
laminate backing film and polyisobutylene adhesive. The white ink contains
acrylic polymers, polyethylene wax, polytetrafluoroethylene,
polyvinylpyrrolidone, sodium dioctyl sulfosuccinate and titanium dioxide.
Keep all transdermal systems within provided containers and dispense one
transdermal system daily.
Apply immediately upon removal from pouch.
Do not store unpouched. Do not store in refrigerators or freezers.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room
Temperature.]
Dosage and Administration: See package insert.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
1-877-446-3679 (1-877-4-INFO-RX)
Mylan.com
M8261:93:30C:R3
Date of Transdermal System Application MM/DD/YYYY | Time Applied | Time Removed | Application Site | Disposal Method |
_AM _PM | _AM _PM | _Right _Left | _Fold & flush _Fold & trash | |
_AM _PM | _AM _PM | _Right _Left | _Fold & flush _Fold & trash |
Principal Display Panel – 2.2 Mg/Hour
Contains: 30 Systems
NDC 0378-8262-93 Rx only
Methylphenidate
Transdermal System CII
2.2 mg/hr
Delivers 20 mg over 9 hours (2.2 mg/hr)
Transdermal system should be worn for approximately 9 hours.
Each transdermal system contains 20.7 mg of methylphenidate.
Active ingredient release is limited; please see recommended dosing in
patient instructions.
For transdermal use only (applied only to skin).
Important: Keep out of the reach of children.
It is important that this product be disposed of properly.
See patient instructions for disposal information.
Pharmacist: Dispense the accompanying
Medication Guide to each patient.
Inactive components: fluoropolymer-coated polyester release liner,
hydrophobic colloidal silica, mineral oil, polyester/ethylene vinyl acetate
laminate backing film and polyisobutylene adhesive. The white ink contains
acrylic polymers, polyethylene wax, polytetrafluoroethylene,
polyvinylpyrrolidone, sodium dioctyl sulfosuccinate and titanium dioxide.
Keep all transdermal systems within provided containers and dispense one
transdermal system daily.
Apply immediately upon removal from pouch.
Do not store unpouched. Do not store in refrigerators or freezers.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room
Temperature.]
Dosage and Administration: See package insert.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
1-877-446-3679 (1-877-4-INFO-RX)
Mylan.com
M8262:93:30C:R3
Date of Transdermal System Application MM/DD/YYYY | Time Applied | Time Removed | Application Site | Disposal Method |
_AM _PM | _AM _PM | _Right _Left | _Fold & flush _Fold & trash | |
_AM _PM | _AM _PM | _Right _Left | _Fold & flush _Fold & trash |
Principal Display Panel – 3.3 Mg/Hour
Contains: 30 Systems
NDC 0378-8263-93 Rx only
Methylphenidate
Transdermal System CII
3.3 mg/hr
Delivers 30 mg over 9 hours (3.3 mg/hr)
Transdermal system should be worn for approximately 9 hours.
Each transdermal system contains 31.1 mg of methylphenidate.
Active ingredient release is limited; please see recommended dosing in
patient instructions.
For transdermal use only (applied only to skin).
Important: Keep out of the reach of children.
It is important that this product be disposed of properly.
See patient instructions for disposal information.
Pharmacist: Dispense the accompanying
Medication Guide to each patient.
Inactive components: fluoropolymer-coated polyester release liner,
hydrophobic colloidal silica, mineral oil, polyester/ethylene vinyl acetate
laminate backing film and polyisobutylene adhesive. The white ink contains
acrylic polymers, polyethylene wax, polytetrafluoroethylene,
polyvinylpyrrolidone, sodium dioctyl sulfosuccinate and titanium dioxide.
Keep all transdermal systems within provided containers and dispense one
transdermal system daily.
Apply immediately upon removal from pouch.
Do not store unpouched. Do not store in refrigerators or freezers.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room
Temperature.]
Dosage and Administration: See package insert.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
1-877-446-3679 (1-877-4-INFO-RX)
Mylan.com
M8263:93:30C:R3
Date of Transdermal System Application MM/DD/YYYY | Time Applied | Time Removed | Application Site | Disposal Method |
_AM _PM | _AM _PM | _Right _Left | _Fold & flush _Fold & trash | |
_AM _PM | _AM _PM | _Right _Left | _Fold & flush _Fold & trash |
* Please review the disclaimer below.