Product Images Azithromycin
View Photos of Packaging, Labels & Appearance
- Figure 1 - azithromycin 01
- Figure 2 - azithromycin 02
- Figure 3 - azithromycin 03
- Figure 4 - azithromycin 04
- Figure 5 - azithromycin 05
- Figure 6 - azithromycin 06
- Figure 7 - azithromycin 07
- Azithromycin Structural Formula - azithromycin 08
- Hospira logo - azithromycin 09
- PRINCIPAL DISPLAY PANEL - 500 mg Vial Label - azithromycin 10
- PRINCIPAL DISPLAY PANEL - 500 mg Vial Tray - azithromycin 11
Product Label Images
The following 11 images provide visual information about the product associated with Azithromycin NDC 0409-0144 by Hospira, Inc., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
PRINCIPAL DISPLAY PANEL - 500 mg Vial Label - azithromycin 10

This is a description of a medication with the NDC code 0409-0144-2T containing azithromycin monohydrate. The medication is in the form of an ADD-Vantage® vial for single use, equivalent to 500 mg of azithromycin. It also contains anhydrous citric acid and sodium hydroxide for pH adjustment. Detailed instructions for use are provided in the package insert for assembling the vial with a flexible diluent container. The diluted solution after preparation contains 2 mg/mL of Azithromycin. It is recommended to store the vial at 20 to 25°C before dilution and to keep the diluted solution stable for 24 hours at room temperature or 7 days under refrigeration. The medication is for intravenous use, and the usual dosage should be followed as per the insert. Distributed by Hospira, Inc. in Lake Forest, IL, USA.*
PRINCIPAL DISPLAY PANEL - 500 mg Vial Tray - azithromycin 11
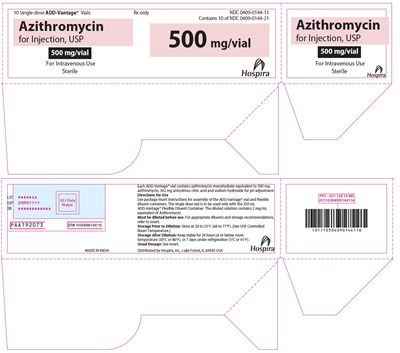
This text provides information about a pharmaceutical product called Azithromycin for Injection, USP, available in single-dose ADD-Vantage vials. Each vial contains azithromycin monohydrate equivalent to 500 mg, along with other components. The vials are intended for intravenous use and must be diluted before administration. Instructions for use and storage recommendations are included. The product is distributed by Hospira, Inc., and the usual dosage information is provided in the insert.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.








