FDA Label for Verapamil Hydrochloride
View Indications, Usage & Precautions
- DESCRIPTION
- CLINICAL PHARMACOLOGY
- MECHANISM OF ACTION
- PHARMACOKINETICS AND METABOLISM
- HEMODYNAMICS AND MYOCARDIAL METABOLISM
- PULMONARY FUNCTION
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- HEART FAILURE
- HYPOTENSION
- ELEVATED LIVER ENZYMES
- ACCESSORY BYPASS TRACT (WOLFF-PARKINSON-WHITE OR LOWN-GANONG-LEVINE)
- ATRIOVENTRICULAR BLOCK
- PATIENTS WITH HYPERTROPHIC CARDIOMYOPATHY (IHSS)
- PRECAUTIONS
- GENERAL
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS: EFFECTS OF OTHER DRUGS ON VERAPAMIL PHARMACOKINETICS
- IVABRADINE
- HMG-COA REDUCTASE INHIBITORS
- BETA BLOCKERS
- CLONIDINE
- DIGITALIS
- ANTIHYPERTENSIVE AGENTS
- ANTIARRHYTHMIC AGENTS
- OTHER
- MAMMALIAN TARGET OF RAPAMYCIN (MTOR) INHIBITORS
- CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR AND DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- ANIMAL PHARMACOLOGY AND/OR ANIMAL TOXICOLOGY
- ADVERSE REACTIONS
- TREATMENT OF ACUTE CARDIOVASCULAR ADVERSE REACTIONS
- OVERDOSAGE
- ESSENTIAL HYPERTENSION
- SPRINKLING THE CAPSULE CONTENTS ON FOOD
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 120 MG CAPSULE BOTTLE LABEL
- PRINCIPAL DISPLAY PANEL - 180 MG CAPSULE BOTTLE LABEL
- PRINCIPAL DISPLAY PANEL - 240 MG CAPSULE BOTTLE LABEL
- PRINCIPAL DISPLAY PANEL - 360 MG CAPSULE BOTTLE LABEL
Verapamil Hydrochloride Product Label
The following document was submitted to the FDA by the labeler of this product Teva Pharmaceuticals Inc. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Description
Verapamil hydrochloride sustained-release capsules are calcium ion influx inhibitors (slow channel blockers or calcium ion antagonists). Verapamil hydrochloride sustained-release capsules are available for oral administration as a 360 mg hard gelatin capsule (lavender cap/yellow body), a 240 mg hard gelatin capsule (dark blue cap/yellow body), a 180 mg hard gelatin capsule (light grey cap/yellow body), and a 120 mg hard gelatin capsule (yellow cap/yellow body). These pellet-filled capsules provide a sustained-release of the drug in the gastrointestinal tract.
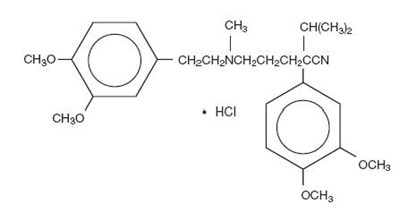
The structural formula of verapamil HCl is given below:
C27H38N2O4•HCl M.W. 491.07
Chemical name: Benzeneacetonitrile, α-[3-[[2-(3,4-dimethoxyphenyl)-ethyl]methylamino]propyl]-3,4-dimethoxy-α-(1-methylethyl), monohydrochloride.
Verapamil HCl is an almost white, crystalline powder, practically free of odor, with a bitter taste. It is soluble in water, chloroform and methanol. Verapamil HCl is not structurally related to other cardioactive drugs.
In addition to verapamil HCl the verapamil hydrochloride sustained-release capsules contain the following inactive ingredients: fumaric acid, talc, sugar spheres, povidone, shellac, gelatin, FD&C red #40, yellow iron oxide, titanium dioxide, methylparaben, propylparaben, silicon dioxide, and sodium lauryl sulfate. In addition, the verapamil hydrochloride sustained-release capsules 240 mg and 360 mg capsules contain FD&C blue #1 and D&C red #28; and the verapamil hydrochloride sustained-release capsules 180 mg contain black iron oxide.
Clinical Pharmacology
Verapamil hydrochloride sustained-release capsules are a calcium ion influx inhibitor (slow channel blockers or calcium ion antagonists) which exerts its pharmacologic effects by modulating the influx of ionic calcium across the cell membrane of the arterial smooth muscle as well as in conductile and contractile myocardial cells.
Normal sinus rhythm is usually not affected by verapamil HCl. However in patients with sick sinus syndrome, verapamil HCl may interfere with sinus node impulse generation and may induce sinus arrest or sinoatrial block. Atrioventricular block can occur in patients without preexisting conduction defects. (See WARNINGS.) Verapamil HCl does not alter the normal atrial action potential or intraventricular conduction time, but depresses amplitude, velocity of depolarization and conduction in depressed atrial fibers. Verapamil HCl may shorten the antegrade effective refractory period of accessory bypass tracts. Acceleration of ventricular rate and/or ventricular fibrillation has been reported in patients with atrial flutter or atrial fibrillation and a coexisting accessory AV pathway following administration of verapamil. (See WARNINGS.)
Verapamil HCl has a local anesthetic action that is 1.6 times that of procaine on an equimolar basis. It is not known whether this action is important at the doses used in man.
Mechanism Of Action
Essential Hypertension
Verapamil HCl exerts antihypertensive effects by decreasing systemic vascular resistance, usually without orthostatic decreases in blood pressure or reflex tachycardia; bradycardia (rate less than 50 beats/minute is uncommon). Verapamil HCl regularly reduces arterial pressure at rest and at a given level of exercise by dilating peripheral arterioles and reducing the total peripheral resistance (afterload) against which the heart works.
Pharmacokinetics And Metabolism
With the immediate release formulations, more than 90% of the orally administered dose is absorbed, and peak plasma concentrations of verapamil are observed 1 to 2 hours after dosing. Because of rapid biotransformation of verapamil during its first pass through the portal circulation, the absolute bioavailability ranges from 20% to 35%. Chronic oral administration of the highest recommended dose (120 mg every 6 hours) resulted in plasma verapamil levels ranging from 125 to 400 ng/mL with higher values reported occasionally. A nonlinear correlation between the verapamil HCl dose administered and verapamil plasma levels does exist.
During initial dose titration with verapamil a relationship exists between verapamil plasma concentrations and the prolongation of the PR interval. However, during chronic administration this relationship may disappear. The quantitative relationship between plasma verapamil concentrations and blood pressure reduction has not been fully characterized.
In a multiple dose pharmacokinetic study, peak concentrations for a single daily dose of verapamil hydrochloride sustained-release capsules 240 mg were approximately 65% of those obtained with an 80 mg t.i.d. dose of the conventional immediate-release tablets, and the 24 hour post-dose concentrations were approximately 30% higher. At a total daily dose of 240 mg, verapamil hydrochloride sustained-release capsules was shown to have a similar extent of verapamil bioavailability based on the AUC-24 as that obtained with the conventional immediate-release tablets. In this same study verapamil hydrochloride sustained-release capsules doses of 120 mg, 240 mg and 360 mg once daily were compared after multiple doses. The ratios of the verapamil and norverapamil AUCs for the verapamil hydrochloride sustained-release capsules 120 mg, 240 mg and 360 mg once daily doses are 1 (565 ng∙hr/mL):3 (1660 ng∙hr/mL):5 (2729 ng∙hr/mL) and 1 (621 ng∙hr/mL):3 (1614 ng∙ hr/mL):4 (2535 ng∙hr/mL) respectively, indicating that the AUC increased non-proportionately with increasing doses.
Food does not affect the extent or rate of the absorption of verapamil from the controlled release verapamil hydrochloride sustained-release capsule. The verapamil hydrochloride sustained-release capsules 240 mg capsule when administered with food had a Cmax of 77 ng/mL which occurred 9.0 hours after dosing, and an AUC(O-inf) of 1387 ng∙hr/mL. Verapamil hydrochloride sustained-release capsules 240 mg under fasting conditions had a Cmax of 77 ng/mL which occurred 9.8 hours after dosing, and an AUC(O-inf) of 1541 ng∙hr/mL.
The bioequivalence of verapamil hydrochloride sustained-release capsule 240 mg, administered as the pellets sprinkled on applesauce and as the intact capsule, was demonstrated in a single-dose, cross-over study in 32 healthy adults. Comparative ratios (sprinkled/intact) of verapamil were 0.95, 1.02, and 1.01 for Cmax, Tmax, and AUC(O-inf) respectively. When the contents of the verapamil hydrochloride sustained-release capsule were administered by sprinkling onto one tablespoonful of applesauce, the rate and extent of verapamil absorption were found to be bioequivalent to the same dose when administered as an intact capsule. Similar results were observed with norverapamil.
The time to reach maximum verapamil concentrations (Tmax) with Verapamil Hydrochloride Sustained-Release Capsules has been found to be approximately 7-9 hours in each of the single dose (fasting), single dose (fed), the multiple dose (steady state) studies and dose proportionality pharmacokinetic studies. Similarly the apparent half-life (t1/2) has been found to be approximately 12 hours independent of dose. Aging may affect the pharmacokinetics of verapamil. Elimination half-life may be prolonged in the elderly.
In healthy man, orally administered verapamil HCl undergoes extensive metabolism in the liver. Twelve metabolites have been identified in plasma; all except norverapamil are present in trace amounts only. Norverapamil can reach steady-state plasma concentrations approximately equal to those of verapamil itself. The biologic activity of norverapamil appears to be approximately 20% that of verapamil.
Approximately 70% of an administered dose of verapamil HCl is excreted as metabolites in the urine and 16% or more in the feces within 5 days. About 3% to 4% is excreted in the urine as unchanged drug. Approximately 90% is bound to plasma proteins. In patients with hepatic insufficiency, metabolism is delayed and elimination half-life prolonged up to 14 to 16 hours (see PRECAUTIONS), the volume of distribution is increased and plasma clearance reduced to about 30% of normal. Verapamil clearance values suggest that patients with liver dysfunction may attain therapeutic verapamil plasma concentrations with one-third of the oral daily dose required for patients with normal liver function.
After four weeks of oral dosing (120 mg q.i.d.), verapamil and norverapamil levels were noted in the cerebrospinal fluid with estimated partition coefficient of 0.06 for verapamil and 0.04 for norverapamil.
In 10 healthy males, administration of oral verapamil (80 mg every 8 hours for 6 days) and a single oral dose of ethanol (0.8 g/kg), resulted in a 17% increase in mean peak ethanol concentrations (106.45 ± 21.40 to 124.23 ± 24.74 mg/dL) compared with placebo. (See PRECAUTIONS-Drug Interactions.)
The area under the blood ethanol concentration versus time curve (AUC over 12 hours) increased by 30% (365.67 ± 93.52 to 475.07 ± 97.24 mg∙hr/dL). Verapamil AUCs were positively correlated (r = 0.71) to increased ethanol blood AUC values.
Geriatric Use: The pharmacokinetics of verapamil GITS were studied after 5 consecutive nights of dosing 180 mg in 30 healthy young (19-43 years) versus 30 healthy elderly (65-80 years) male and female subjects. Older subjects had significantly higher mean verapamil Cmax, Cmin and AUC (0-24h) compared to younger subjects. Older subjects had mean AUCs that were approximately 1.7-2.0 times higher than those of younger subjects as well as a longer average verapamil t1/2 (approximately 20 hr vs 13 hr).
Hemodynamics And Myocardial Metabolism
Verapamil HCl reduces afterload and myocardial contractility. Improved left ventricular diastolic function in patients with IHSS and those with coronary heart disease has also been observed with verapamil HCl therapy. In most patients, including those with organic cardiac disease, the negative inotropic action of verapamil HCl is countered by reduction of afterload and cardiac index is usually not reduced. In patients with severe left ventricular dysfunction however, (e.g., pulmonary wedge pressure above 20 mm Hg or ejection fraction lower than 30%), or in patients on beta-adrenergic blocking agents or other cardio-depressant drugs, deterioration of ventricular function may occur. (See Drug Interactions.)
Pulmonary Function
Verapamil HCl does not induce broncho-constriction and hence, does not impair ventilatory function.
Indications And Usage
Verapamil hydrochloride sustained-release capsules are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including this drug.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Contraindications
Verapamil HCl is contraindicated in:
- Severe left ventricular dysfunction. (See WARNINGS.)
- Hypotension (less than 90 mm Hg systolic pressure) or cardiogenic shock.
- Sick sinus syndrome (except in patients with a functioning artificial ventricular pacemaker).
- Second- or third-degree AV block (except in patients with a functioning artificial ventricular pacemaker).
- Patients with atrial flutter or atrial fibrillation and an accessory bypass tract (e.g., Wolff-Parkinson-White, Lown-Ganong-Levine syndromes). (See WARNINGS.)
- Patients with known hypersensitivity to verapamil hydrochloride.
Heart Failure
Verapamil has a negative inotropic effect which, in most patients, is compensated by its afterload reduction (decreased systemic vascular resistance) properties without a net impairment of ventricular performance. In clinical experience with 4,954 patients, 87 (1.8%) developed congestive heart failure or pulmonary edema. Verapamil should be avoided in patients with severe left ventricular dysfunction (e.g., ejection fraction less than 30% or moderate to severe symptoms of cardiac failure) and in patients with any degree of ventricular dysfunction if they are receiving a beta-adrenergic blocker. (See DRUG INTERACTIONS.) Patients with milder ventricular dysfunction should, if possible, be controlled with optimum doses of digitalis and/or diuretics before verapamil treatment (note interactions with digoxin under: PRECAUTIONS).
Hypotension
Occasionally, the pharmacologic action of verapamil may produce a decrease in blood pressure below normal levels which may result in dizziness or symptomatic hypotension. The incidence of hypotension observed in 4,954 patients enrolled in clinical trials was 2.5%. In hypertensive patients, decreases in blood pressure below normal are unusual. Tilt table testing (60 degrees) was not able to induce orthostatic hypotension.
Elevated Liver Enzymes
Elevations of transaminases with and without concomitant elevations in alkaline phosphatase and bilirubin have been reported. Such elevations have sometimes been transient and may disappear even in the face of continued verapamil treatment. Several cases of hepatocellular injury related to verapamil have been proven by rechallenge; half of these had clinical symptoms (malaise, fever, and/or right upper quadrant pain) in addition to elevations of SGOT, SGPT and alkaline phosphatase. Periodic monitoring of liver function in patients receiving verapamil is therefore prudent.
Accessory Bypass Tract (Wolff-Parkinson-White Or Lown-Ganong-Levine)
Some patients with paroxysmal and/or chronic atrial flutter or atrial fibrillation and a coexisting accessory AV pathway have developed increased antegrade conduction across the accessory pathway bypassing the AV node, producing a very rapid ventricular response or ventricular fibrillation after receiving intravenous verapamil (or digitalis). Although a risk of this occurring with oral verapamil has not been established, such patients receiving oral verapamil may be at risk and its use in these patients is contraindicated. (See CONTRAINDICATIONS.)
Treatment is usually DC-cardioversion. Cardioversion has been used safely and effectively after oral verapamil.
Atrioventricular Block
The effect of verapamil on AV conduction and the SA node may lead to asymptomatic first-degree AV block and transient bradycardia, sometimes accompanied by nodal escape rhythms. PR interval prolongation is correlated with verapamil plasma concentrations, especially during the early titration phase of therapy. Higher degrees of AV block, however, were infrequently (0.8%) observed.
Marked first-degree block or progressive development to second- or third-degree AV block requires a reduction in dosage or, in rare instances, discontinuation of verapamil HCl and institution of appropriate therapy depending upon the clinical situation.
Patients With Hypertrophic Cardiomyopathy (Ihss)
In 120 patients with hypertrophic cardiomyopathy (most of them refractory or intolerant to propranolol) who received therapy with verapamil at doses up to 720 mg/day, a variety of serious adverse effects were seen. Three patients died in pulmonary edema; all had severe left ventricular outflow obstruction and a past history of left ventricular dysfunction. Eight other patients had pulmonary edema and/or severe hypotension; abnormally high (over 20 mm Hg) capillary wedge pressure and a marked left ventricular outflow obstruction were present in most of these patients. Concomitant administration of quinidine (see Drug Interactions) preceded the severe hypotension in 3 of the 8 patients (2 of whom developed pulmonary edema). Sinus bradycardia occurred in 11% of the patients, second-degree AV block in 4% and sinus arrest in 2%. It must be appreciated that this group of patients had a serious disease with a high mortality rate. Most adverse effects responded well to dose reduction and only rarely did verapamil have to be discontinued.
Precautions
THE CONTENTS OF THE VERAPAMIL HYDROCHLORIDE SUSTAINED-RELEASE CAPSULES SHOULD NOT BE CRUSHED OR CHEWED. VERAPAMIL HYDROCHLORIDE SUSTAINED-RELEASE CAPSULES ARE TO BE SWALLOWED WHOLE OR THE ENTIRE CONTENTS OF THE CAPSULE SPRINKLED ONTO APPLESAUCE (see DOSAGE AND ADMINISTRATION).
General
Use in Patients with Impaired Hepatic Function
Since verapamil is highly metabolized by the liver, it should be administered cautiously to patients with impaired hepatic function. Severe liver dysfunction prolongs the elimination half-life of immediate-release verapamil to about 14 to 16 hours; hence, approximately 30% of the dose given to patients with normal liver function should be administered to these patients. Careful monitoring for abnormal prolongation of the PR interval or other signs of excessive pharmacologic effects (see OVERDOSAGE) should be carried out.
Use in Patients with Attenuated (Decreased) Neuromuscular Transmission
It has been reported that verapamil decreases neuromuscular transmission in patients with Duchenne's muscular dystrophy, and that verapamil prolongs recovery from the neuromuscular blocking agent vecuronium and causes a worsening of myasthenia gravis. It may be necessary to decrease the dosage of verapamil when it is administered to patients with attenuated neuromuscular transmission.
Use in Patients with Impaired Renal Function
About 70% of an administered dose of verapamil is excreted as metabolites in the urine. Until further data are available, verapamil should be administered cautiously to patients with impaired renal function. These patients should be carefully monitored for abnormal prolongation of the PR interval or other signs of overdosage. (See OVERDOSAGE.)
Information For Patients
When the sprinkle method of administration is prescribed, details of the proper technique should be explained to the patient. (See DOSAGE AND ADMINISTRATION.)
Drug Interactions: Effects Of Other Drugs On Verapamil Pharmacokinetics
In vitro metabolic studies indicate that verapamil is metabolized by cytochrome P450, CYP3A4, CYP1A2, and CYP2C. Clinically significant interactions have been reported with inhibitors of CYP3A4 (e.g., erythromycin, ritonavir) causing elevation of plasma levels of verapamil while inducers of CYP3A4 (e.g., rifampin) have caused a lowering of plasma levels of verapamil. Hypotension, bradyarrhythmias, and lactic acidosis have been observed in patients receiving concurrent telithromycin, an antibiotic in the ketolide class of antibiotics.
Ivabradine
Concurrent use of verapamil increases exposure to ivabradine and may exacerbate bradycardia and conductions disturbances. Avoid concomitant use of ivabradine and verapamil.
Hmg-Coa Reductase Inhibitors
The use of HMG-CoA reductase inhibitors that are CYP3A4 substrates in combination with verapamil has been associated with reports of myopathy/rhabdomyolysis.
Co-administration of multiple doses of 10 mg of verapamil with 80 mg simvastatin resulted in exposure to simvastatin 2.5-fold that following simvastatin alone. Limit the dose of simvastatin in patients on verapamil to 10 mg daily. Limit the daily dose of lovastatin to 40 mg. Lower starting and maintenance doses of other CYP3A4 substrates (e.g., atorvastatin) may be required as verapamil may increase the plasma concentration of these drugs.
Beta Blockers
Concomitant therapy with beta-adrenergic blockers and verapamil may result in additive negative effects on heart rate, atrioventricular conduction, and/or cardiac contractility. The combination of sustained-release verapamil and beta-adrenergic blocking agents has not been studied. However, there have been reports of excess bradycardia and AV block, including complete heart block, when the combination has been used for the treatment of hypertension.
For hypertensive patients, the risk of combined therapy may outweigh the potential benefits. The combination should be used only with caution and close monitoring.
Asymptomatic bradycardia (36 beats/min) with a wandering atrial pacemaker has been observed in a patient receiving concomitant timolol (a beta-adrenergic blocker) eyedrops and oral verapamil.
A decrease in metoprolol clearance has been reported when verapamil and metoprolol were administered together. A similar effect has not been observed when verapamil and atenolol are given together.
Clonidine
Sinus bradycardia resulting in hospitalization and pacemaker insertion has been reported in association with the use of clonidine concurrently with verapamil. Monitor heart rate in patients receiving concomitant verapamil and clonidine.
Digitalis
Consider reducing digoxin dose when verapamil and digoxin are to be given together. Monitor digoxin level periodically during therapy. Chronic verapamil treatment can increase serum digoxin levels by 50% to 75% during the first week of therapy, and this can result in digitalis toxicity. In patients with hepatic cirrhosis the influence of verapamil on digoxin pharmacokinetics is magnified. Verapamil may reduce total body clearance and extrarenal clearance of digoxin by 27% and 29%, respectively. If digoxin toxicity is suspected, suspend or discontinue digoxin therapy.
In previous clinical trials with other verapamil formulations related to the control of ventricular response in patients taking digoxin who had atrial fibrillation or atrial flutter, ventricular rates below 50/min at rest occurred in 15% of patients, and asymptomatic hypotension occurred in 5% of patients.
Antihypertensive Agents
Verapamil administered concomitantly with oral antihypertensive agents (e.g., vasodilators, angiotensin-converting enzyme inhibitors, diuretics, beta blockers) will usually have an additive effect on lowering blood pressure. Patients receiving these combinations should be appropriately monitored. Concomitant use of agents that attenuate alpha-adrenergic function with verapamil may result in reduction in blood pressure that is excessive in some patients. Such an effect was observed in one study following the concomitant administration of verapamil and prazosin.
Antiarrhythmic Agents
Disopyramide: Until data on possible interactions between verapamil and disopyramide phosphate are obtained, disopyramide should not be administered within 48 hours before or 24 hours after verapamil administration.
Flecainide: A study in healthy volunteers showed that the concomitant administration of flecainide and verapamil may have additive effects on myocardial contractility, AV conduction, and repolarization. Concomitant therapy with flecainide and verapamil may result in additive negative inotropic effect and prolongation of atrioventricular conduction.
Quinidine: In a small number of patients with hypertrophic cardiomyopathy (IHSS), concomitant use of verapamil and quinidine resulted in significant hypotension. Until further data are obtained, combined therapy of verapamil and quinidine in patients with hypertrophic cardiomyopathy should probably be avoided.
The electrophysiological effects of quinidine and verapamil on AV conduction were studied in 8 patients. Verapamil significantly counteracted the effects of quinidine on AV conduction. There has been a report of increased quinidine levels during verapamil therapy.
Nitrates: Verapamil has been given concomitantly with short- and long-acting nitrates without any undesirable drug interactions. The pharmacologic profile of both drugs and the clinical experience suggest beneficial interactions.
Alcohol: Verapamil has been found to significantly inhibit ethanol elimination resulting in elevated blood ethanol concentrations that may prolong the intoxicating effects of alcohol. (See CLINICAL PHARMACOLOGY - Pharmacokinetics and Metabolism.)
Other
Aspirin: In a few reported cases, coadministration of verapamil with aspirin has led to increased bleeding times greater than observed with aspirin alone.
Cimetidine: The interaction between cimetidine and chronically administered verapamil has not been studied. Variable results on clearance have been obtained in acute studies of healthy volunteers; clearance of verapamil was either reduced or unchanged.
Grapefruit Juice: Grapefruit juice may significantly increase concentrations of verapamil. Grapefruit juice given to nine healthy volunteers increased S- and R- verapamil AUC0-12 by 36% and 28%, respectively. Steady state Cmax and Cmin of S-verapamil increased by 57% and 16.7%, respectively with grapefruit juice compared to control. Similarly, Cmax and Cmin of R-verapamil increased by 40% and 13%, respectively. Grapefruit juice did not affect half-life, nor was there a significant change in AUC0-12 ratio R/S compared to control. Grapefruit juice did not cause a significant difference in the PK of norverapamil. This increase in verapamil plasma concentration is not expected to have any clinical consequences.
Lithium: Pharmacokinetic and pharmacodynamic interactions between oral verapamil and lithium have been reported. The former may result in a lowering of serum lithium levels in patients receiving chronic stable oral lithium therapy. The latter may result in an increased sensitivity to the effects of lithium. Patients receiving both drugs must be monitored carefully.
Carbamazepine: Verapamil therapy may increase carbamazepine concentrations during combined therapy. This may produce carbamazepine side effects such as diplopia, headache, ataxia, or dizziness.
Rifampin: Therapy with rifampin may markedly reduce oral verapamil bioavailability.
Phenobarbital: Phenobarbital therapy may increase verapamil clearance.
Cyclosporine: Verapamil therapy may increase serum levels of cyclosporine.
Theophylline: Verapamil may inhibit the clearance and increase the plasma levels of theophylline.
Inhalation Anesthetics: Animal experiments have shown that inhalation anesthetics depress cardiovascular activity by decreasing the inward movement of calcium ions. When used concomitantly, inhalation anesthetics and calcium antagonists, such as verapamil, should be titrated carefully to avoid excessive cardiovascular depression.
Neuromuscular Blocking Agents: Clinical data and animal studies suggest that verapamil may potentiate the activity of neuromuscular blocking agents (curare-like and depolarizing). It may be necessary to decrease the dose of verapamil and/or the dose of the neuromuscular blocking agent when the drugs are used concomitantly.
Mammalian Target Of Rapamycin (Mtor) Inhibitors
In a study of 25 healthy volunteers with co-administration of verapamil with sirolimus, whole blood sirolimus Cmax and AUC were increased 130% and 120%, respectively. Plasma S (-) verapamil Cmax and AUC were both increased 50%. Co-administration of verapamil with everolimus in 16 healthy volunteers increased the Cmax and AUC of everolimus by 130% and 250%, respectively. With concomitant use of mTOR inhibitors (e.g., sirolimus, temsirolimus, and everolimus) and verapamil, consider appropriate dose reductions of both medications.
Carcinogenesis, Mutagenesis, Impairment Of Fertility
An 18-month toxicity study in rats, at a low multiple (6 fold) of the maximum recommended human dose, and not the maximum tolerated dose, did not suggest a tumorigenic potential. There was no evidence of a carcinogenic potential of verapamil administered in the diet of rats for two years at doses of 10, 35 and 120 mg/kg per day or approximately 1x, 3.5x and 12x, respectively, the maximum recommended human daily dose (480 mg per day or 9.6 mg/kg/day).
Verapamil was not mutagenic in the Ames test in 5 test strains at 3 mg per plate, with or without metabolic activation.
Studies in female rats at daily dietary doses up to 5.5 times (55 mg/kg/day) the maximum recommended human dose did not show impaired fertility. Effects on male fertility have not been determined.
Pregnancy
Pregnancy Category C. Reproduction studies have been performed in rabbits and rats at oral doses up to 1.5 (15 mg/kg/day) and 6 (60 mg/kg/day) times the maximum recommended human daily dose, respectively, and have revealed no evidence of teratogenicity. In the rat, however, this multiple of the human dose was embryocidal and retarded fetal growth and development, probably because of adverse maternal effects reflected in reduced weight gains of the dams. This oral dose has also been shown to cause hypotension in rats. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Verapamil crosses the placental barrier and can be detected in umbilical vein blood at delivery.
Labor And Delivery
It is not known whether the use of verapamil during labor or delivery has immediate or delayed adverse effects on the fetus, or whether it prolongs the duration of labor or increases the need for forceps delivery or other obstetric intervention. Such adverse experiences have not been reported in the literature, despite a long history of use of verapamil HCl in Europe in the treatment of cardiac side effects of beta-adrenergic agonist agents used to treat premature labor.
Nursing Mothers
Verapamil is excreted in human milk. Because of the potential for adverse reactions in nursing infants from verapamil, nursing should be discontinued while verapamil is administered.
Pediatric Use
Safety and efficacy of verapamil in children below the age of 18 years have not been established.
Geriatric Use
Clinical studies of verapamil did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Aging may affect the pharmacokinetics of verapamil. Elimination half-life may be prolonged in the elderly (see CLINICAL PHARMACOLOGY, Pharmacokinetics and Metabolism).
Verapamil is highly metabolized by the liver, and about 70% of the administered dose is excreted as metabolites in the urine. Clinical circumstances, some of which may be more common in the elderly, such as hepatic or renal impairment, should be considered (see PRECAUTIONS, General). In general, lower initial doses of verapamil hydrochloride sustained-release capsules may be warranted in the elderly (see DOSAGE AND ADMINISTRATION).
Animal Pharmacology And/Or Animal Toxicology
In chronic animal toxicology studies verapamil caused lenticular and/or suture line changes at 30 mg/kg/day or greater and frank cataracts at 62.5 mg/kg/day or greater in the beagle dog but not the rat. Development of cataracts due to verapamil has not been reported in man.
Adverse Reactions
Serious adverse reactions are uncommon when verapamil HCl therapy is initiated with upward dose titration within the recommended single and total daily dose. See WARNINGS for discussion of heart failure, hypotension, elevated liver enzymes, AV block, and rapid ventricular response. Reversible (upon discontinuation of verapamil) non-obstructive, paralytic ileus has been infrequently reported in association with the use of verapamil.
In clinical trials involving 285 hypertensive patients on verapamil hydrochloride sustained-release capsules for greater than 1 week the following adverse reactions were reported in greater than 1.0% of the patients:
| Constipation | 7.4% |
| Headache | 5.3% |
| Dizziness | 4.2% |
| Lethargy | 3.2% |
| Dyspepsia | 2.5% |
| Rash | 1.4% |
| Ankle Edema | 1.4% |
| Sleep Disturbance | 1.4% |
| Myalgia | 1.1% |
In clinical trials of other formulations of verapamil HCl (N=4,954) the following reactions have occurred at rates greater than 1.0%:
| Constipation | 7.3% |
| Dizziness | 3.3% |
| Nausea | 2.7% |
| Hypotension | 2.5% |
| Edema | 1.9% |
| Headache | 2.2% |
| Rash | 1.2% |
| CHF/Pulmonary Edema | 1.8% |
| Fatigue | 1.7% |
| Bradycardia (HR<50/min) | 1.4% |
| AV block-total 1°, 2°, 3° | 1.2% |
| 2° and 3° | 0.8% |
| Flushing | 0.6% |
| Elevated Liver Enzymes (See WARNINGS) |
In clinical trials related to the control of ventricular response in digitalized patients who had atrial fibrillation or atrial flutter, ventricular rate below 50/min at rest occurred in 15% of patients and asymptomatic hypotension occurred in 5% of patients.
The following reactions, reported in 1.0% or less of patients, occurred under conditions (open trials, marketing experience) where a causal relationship is uncertain; they are listed to alert the physician to a possible relationship:
Cardiovascular: angina pectoris, atrioventricular dissociation, chest pain, claudication, myocardial infarction, palpitations, purpura (vasculitis), syncope.
Digestive System: diarrhea, dry mouth, gastrointestinal distress, gingival hyperplasia.
Hemic and Lymphatic: ecchymosis or bruising.
Nervous System: cerebrovascular accident, confusion, equilibrium disorders, extrapyramidal symptoms, insomnia, muscle cramps, paresthesia, psychotic symptoms, shakiness, somnolence.
Respiratory: dyspnea.
Skin: arthralgia and rash, exanthema, hair loss, hyperkeratosis, maculae, sweating, urticaria, Stevens-Johnson syndrome, erythema multiforme.
Special Senses: blurred vision, tinnitus.
Urogenital: gynecomastia, impotence, increased urination, spotty menstruation.
Treatment Of Acute Cardiovascular Adverse Reactions
The frequency of cardiovascular adverse reactions which require therapy is rare; hence, experience with their treatment is limited. Whenever severe hypotension or complete AV block occurs following oral administration of verapamil, the appropriate emergency measures should be applied immediately, e.g., intravenously administered isoproterenol HCl, levarterenol bitartrate, atropine (all in the usual doses), or calcium gluconate (10% solution). In patients with hypertrophic cardiomyopathy (IHSS), alpha-adrenergic agents (phenylephrine, metaraminol bitartrate or methoxamine) should be used to maintain blood pressure, and isoproterenol and levarterenol should be avoided. If further support is necessary, inotropic agents (dopamine or dobutamine) may be administered. Actual treatment and dosage should depend on the severity and the clinical situation and the judgment and experience of the treating physician.
To report SUSPECTED ADVERSE EVENTS, contact Actavis at 1-800-272-5525 or FDA at 1-800-FDA-1088 or http://www.fda.gov/ for voluntary reporting of adverse reactions.
Overdosage
There is no specific antidote for verapamil overdosage; treatment should be supportive. Delayed pharmacodynamic consequences may occur with sustained-release formulations, and patients should be observed for at least 48 hours, preferably under continuous hospital care. Reported effects include hypotension, bradycardia, cardiac conduction defects, arrhythmias, hyperglycemia, and decreased mental status. In addition, there have been literature reports of non-cardiogenic pulmonary edema in patients taking large overdoses of verapamil (up to approximately 9g).
In acute overdosage, gastrointestinal decontamination with cathartics and whole bowel irrigation should be considered. Calcium, inotropes (i.e., isoproterenol, dopamine, and glucagon), atropine, vasopressors (i.e., norepinephrine, and epinephrine), and cardiac pacing have been used with variable results to reverse hypotension and myocardial depression. In a few reported cases, overdose with calcium channel blockers that was initially refractory to atropine became more responsive to this treatment when the patients received large doses (close to 1g/hour for more than 24 hours) of calcium chloride. Calcium chloride is preferred to calcium gluconate since it provides 3 times more calcium per volume. Asystole should be handled by the usual measures including cardiopulmonary resuscitation. Verapamil cannot be removed by hemodialysis.
Essential Hypertension
The dose of verapamil hydrochloride sustained-release capsules should be individualized by titration. The usual daily dose of sustained-release verapamil, verapamil hydrochloride sustained-release capsules, in clinical trials has been 240 mg given by mouth once daily in the morning. However, initial doses of 120 mg a day may be warranted in patients who may have an increased response to verapamil (e.g., elderly, small people, etc.). Upward titration should be based on therapeutic efficacy and safety evaluated approximately 24 hours after dosing. The antihypertensive effects of verapamil hydrochloride sustained-release capsules are evident within the first week of therapy.
If adequate response is not obtained with 120 mg of verapamil hydrochloride sustained-release capsules, the dose may be titrated upward in the following manner:
(a) 180 mg in the morning.
(b) 240 mg in the morning.
(c) 360 mg in the morning.
(d) 480 mg in the morning.
Verapamil hydrochloride sustained-release capsules are for once-a-day administration. When switching from immediate-release verapamil to verapamil hydrochloride sustained-release capsules, the same total daily dose of verapamil hydrochloride sustained-release capsules can be used.
As with immediate-release verapamil, dosages of verapamil hydrochloride sustained-release capsules should be individualized and titration may be needed in some patients.
Sprinkling The Capsule Contents On Food
Verapamil hydrochloride sustained-release capsules may also be administered by carefully opening the capsule and sprinkling the pellets on a spoonful of applesauce. The applesauce should be swallowed immediately without chewing and followed with a glass of cool water to ensure complete swallowing of the pellets. The applesauce used should not be hot, and it should be soft enough to be swallowed without chewing. Any pellet/applesauce mixture should be used immediately and not stored for future use. Subdividing the contents of a verapamil hydrochloride sustained-release capsule is not recommended.
How Supplied
Verapamil hydrochloride sustained-release capsules are supplied in four dosage strengths:
| 120 mg - | Two-piece, size 2 hard gelatin capsule (yellow cap/yellow body), printed with 60274 and 120 mg in black ink, supplied as follows: |
| NDC 0591-2880-01 - Bottle of 100s | |
| 180 mg - | Two-piece, size 1 elongated hard gelatin capsule (light grey cap/yellow body), printed with 60274 and 180 mg in black ink, supplied as follows: |
| NDC 0591-2882-01 - Bottle of 100s | |
| 240 mg - | Two-piece, size 0 hard gelatin capsule (dark blue cap/yellow body), printed with 60274 and 240 mg in black ink, supplied as follows: |
| NDC 0591-2884-01 - Bottle of 100s | |
| 360 mg - | Two-piece, size 00 hard gelatin capsule (lavender cap/yellow body), printed with 60274 and 360 mg in black ink, supplied as follows: |
| NDC 0591-2886-01 - Bottle of 100s |
Principal Display Panel - 120 Mg Capsule Bottle Label
NDC 0591-2880-01
120
mg
Verapamil
Hydrochloride
Sustained-Release
Capsules
Actavis
100 Capsules
Rx Only
Principal Display Panel - 180 Mg Capsule Bottle Label
NDC 0591-2882-01
180
mg
Verapamil
Hydrochloride
Sustained-Release
Capsules
Actavis
100 Capsules
Rx Only
Principal Display Panel - 240 Mg Capsule Bottle Label
NDC 0591-2884-01
240
mg
Verapamil
Hydrochloride
Sustained-Release
Capsules
Actavis
100 Capsules
Rx Only
Principal Display Panel - 360 Mg Capsule Bottle Label
NDC 0591-2886-01
360
mg
Verapamil
Hydrochloride
Sustained-Release
Capsules
Actavis
100 Capsules
Rx Only
* Please review the disclaimer below.