Product Images Ultracare Anesthetic Gel
View Photos of Packaging, Labels & Appearance
- Benzocaine 20% w/v - API
- alert - Allergy Alert
- keep out of reach of children - Children
- consult - Consult
- Do not use in patients with history of ypersensitivity to any ester-type local anesthetics. Do not use the product for teething or in infants and children younger than 2 years - DNU
- dnu - DNU2
- Dose admin - DoseAdmin
- facts - DrugFacts1
- facts - DrugFacts2
- Inactive - Inactive
- Reduce pain or discomfort caused by minor dental procedures • minor gum injury • canker sores • sore throat • minor mouth or gum irritations caused by dentures or orthodontic appliances. - Intended Use
- lable - label
- storage - storage
- warnings - warnings
Product Label Images
The following 14 images provide visual information about the product associated with Ultracare Anesthetic Gel NDC 10129-072 by The Belport Company, Inc. Dba Gingi-pak, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Benzocaine 20% w/v - API

This product contains 20% Benzocaine as its active ingredient, which functions as an oral anesthetic to provide relief from pain or discomfort in the mouth.*
alert - Allergy Alert

This text contains an allergy alert advising individuals not to use the product if they have a history of allergy to any "caine" local anesthetics or FD&C Red #40. It also warns to avoid contact with eyes.*
keep out of reach of children - Children
This is a safety warning advising individuals to ensure that a certain item or product is not accessible to children to prevent any potential harm or danger.*
consult - Consult

This text appears to be a warning label advising users to consult a doctor or pharmacist before using the product if they have severe injuries, infections, or conditions in specific areas mentioned. It also instructs users to seek medical attention if they experience severe sore throat lasting more than 2 days, develop fever, headache, rash, nausea, or vomiting, have a mouth sore lasting more than 10 days, or if irritation, pain, or redness worsens.*
Do not use in patients with history of ypersensitivity to any ester-type local anesthetics. Do not use the product for teething or in infants and children younger than 2 years - DNU

This text provides important safety information about the use of a specific product, cautioning against its use in patients with a history of hypersensitivity to certain types of local anesthetics. It also specifies that the product should not be used for teething or in infants and children younger than 2 years old.*
Dose admin - DoseAdmin

This text provides instructions for the proper use of a local anesthetic on mucosa. It advises drying the area before application to enhance effectiveness, using cotton rolls or saliva ejectors to remove excess saliva, and avoiding cross-contamination between patients. The dosage should not exceed the amount needed for anesthesia, and the anesthetic should be applied to the affected area, remaining in place for at least 1 minute before spitting. The product is recommended for adults and children aged 2 years and older, with children under 2 years advised to consult a dentist or doctor before use. It can be used up to 4 times daily or as directed by a healthcare professional, and users should not exceed the recommended dosage.*
Inactive - Inactive

This product contains the following inactive ingredients: Polyethylene Glycol 400 (PEG 400) NF, Polyethylene Glycol 3350 (3550) NF, Sodium Saccharin, artificial flavor, and FD&C Red #40.*
Reduce pain or discomfort caused by minor dental procedures • minor gum injury • canker sores • sore throat • minor mouth or gum irritations caused by dentures or orthodontic appliances. - Intended Use

This product is designed to reduce pain or discomfort caused by various dental procedures and minor oral irritations such as canker sores, sore throats, and gum injuries. It can also help with mouth or gum irritations from dentures or orthodontic appliances. The directions involve using this product to provide anesthesia to the mucous membranes of the oropharynx, minimizing pain during ulcers, needle punctures, scaling procedures, and other dental applications. It can also assist with patients who have an excessive gag reflex during impressions or intraoral radiographs.*
lable - label

This is a product description for a topical anesthetic gel called Ultra Care, containing 20% Benzocaine Oral Anesthetic. It is manufactured by Ultradent Products Inc. in the USA. For more information, visit ultradent.com or contact 800.552.5512.*
storage - storage

This product should be stored in a place with a temperature below 40° C (104° F) to prevent damage. The net content of the product is 1 oz. (30 g).*
warnings - warnings
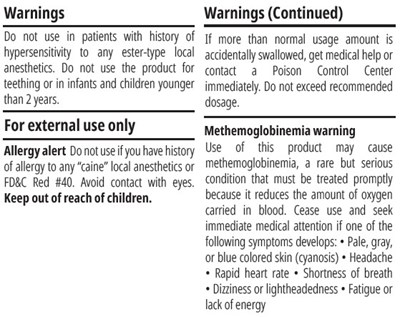
This text provides warnings and instructions for a specific product, advising on precautions and potential risks. It highlights contraindications for patients with hypersensitivity to local anesthetics, caution regarding usage in infants and children under 2 years, and recommendations for external use only. Additionally, it emphasizes the risks of allergic reactions, the importance of avoiding eye contact, keeping the product away from children, and the possibility of methemoglobinemia, a serious condition that can arise from its usage. Symptoms to watch for and immediate medical attention needed in case of such adverse reactions are also outlined.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.


