FDA Label for Alcohol Prep Pad
View Indications, Usage & Precautions
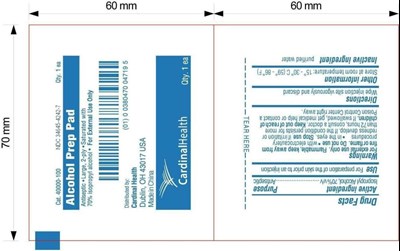
Alcohol Prep Pad Product Label
The following document was submitted to the FDA by the labeler of this product Jiangsu Province Jianerkang Medical Dressing Co. ,ltd.. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Package Label.Principal Display Panel
Cat. 40000-100 NDC 34645-4242-7 Qty.1ea
Alcohol Prep
Pad
Antiseptic * Large, 2-ply * Saturated with 70% isopropyl alcohol
* For External Use Only
Distributed by:
Cardinal Health
Dublin, OH
43017 USA
Made in China
Image of Pouch Label
Otc - Active Ingredient
Active ingredient
Isopropyl Alcohol, 70% v/v
Otc - Purpose
Purpose
Antiseptic
Otc - When Using
Use
For preparation of skin prior to injection
Warnings
Warnings
For external use only.
Flammable, keep away from fire or flame.
Otc - Do Not Use
Do not use
with electrocautery procedures, in the eyes.
Otc - Stop Use
Stop useif irritation or redness develop. If the condition persists for more than 72 hours, consult a doctor.
Otc - Keep Out Of Reach Of Children
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center right way.
Indications & Usage
Directions
Wipe injection site vigorously and discard
Storage And Handling
Other information
Store at room temperature: 15。- 30。C(59。-86。F)
Inactive Ingredient
Inactive ingredient
purified water
* Please review the disclaimer below.