Product Images Sacubitril And Valsartan
View Photos of Packaging, Labels & Appearance
- figure1 - sacubitrilvalsartan figure1
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 49 mg/51 mg - cold form Blister (1 x 10's count) - sacubitrilvalsartan figure10
- sacubitrilvalsartan-figure11.jpg - sacubitrilvalsartan figure11
- sacubitrilvalsartan-figure12.jpg - sacubitrilvalsartan figure12
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 97 mg/103 mg - cold form Blister (1 x 10's count) - sacubitrilvalsartan figure13
- sacubitrilvalsartan-figure14 - sacubitrilvalsartan figure14
- sacubitrilvalsartan-figure15 - sacubitrilvalsartan figure15
- sacubitrilvalsartan-figure16 - sacubitrilvalsartan figure16
- figure2 - sacubitrilvalsartan figure2
- sacubitrilvalsartan-figure3 - sacubitrilvalsartan figure3
- sacubitrilvalsartan-figure4 - sacubitrilvalsartan figure4
- sacubitrilvalsartan-figure5.jpg - sacubitrilvalsartan figure5
- sacubitrilvalsartan-figure6.jpg - sacubitrilvalsartan figure6
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 24 mg/26 mg - cold form Blister (1 x 10's count) - sacubitrilvalsartan figure7
- sacubitrilvalsartan-figure8.jpg - sacubitrilvalsartan figure8
- sacubitrilvalsartan-figure9.jpg - sacubitrilvalsartan figure9
- sacubitrilvalsartan-structure - sacubitrilvalsartan structure
Product Label Images
The following 17 images provide visual information about the product associated with Sacubitril And Valsartan NDC 42385-930 by Laurus Labs Limited, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 49 mg/51 mg - cold form Blister (1 x 10's count) - sacubitrilvalsartan figure10

sacubitrilvalsartan-figure12.jpg - sacubitrilvalsartan figure12

This is the packaging information for Sacubitril and Valsartan tablets manufactured by Laurus Labs. Each film-coated tablet contains 97 mg of sacubitil and 103 mg of valsartan. The usual dosage information should be referred to in the prescribing information. The tablets are for institutional use only and are available as 100 (10 x 10) unit-dose tablets. It is advised to store the tablets between 20° to 25°C (68° to 77°F) with excursions permitted between 15 to 30°C (59° to 86°F) as per USP Controlled Room Temperature guidelines. The drugs should be protected from moisture and kept out of the reach of children. The tablets should be used as per prescription and regulatory guidelines.*
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 97 mg/103 mg - cold form Blister (1 x 10's count) - sacubitrilvalsartan figure13

sacubitrilvalsartan-figure14 - sacubitrilvalsartan figure14

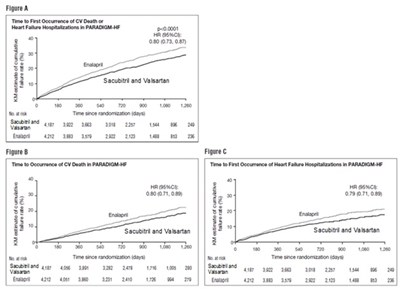
This text provides information about the Mean Number of Events Over Time for the Primary Composite Endpoint of Total Heart Failure Hospitalizations and Cardiovascular Death in the PARAGON-HE study. It includes data on Sacubitril and Valsartan with a mean number of events per 100 patients being 8. The graph presented shows the time since randomization in days.*
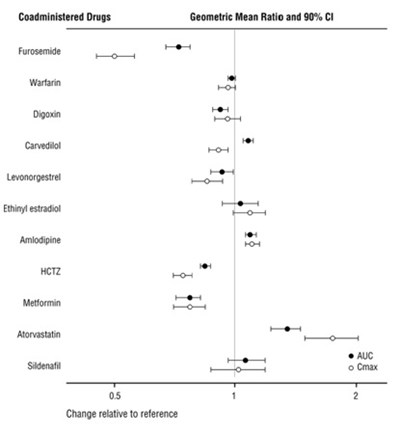
sacubitrilvalsartan-figure4 - sacubitrilvalsartan figure4

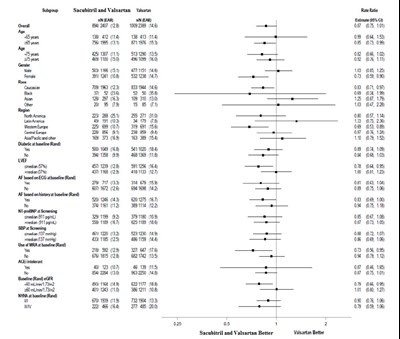
This text appears to be a table showing data related to the percentage of various factors in relation to Sacubitril and Valsartan as well as Enalapril, such as age groups, gender, weight, race, NYHA class, diabetes status, systolic blood pressure, ejection fraction, atrial fibrillation, medication history, heart failure diagnosis, cause of heart failure, presence of ICD, and hazard ratios. The table provides information on how these factors compare between the groups receiving Sacubitril and Valsartan versus Enalapril. It seems to be a clinical or medical study comparing the effectiveness of these medications for heart failure. The study indicates that Sacubitril and Valsartan may be more beneficial compared to Enalapril based on various factors analyzed.*
sacubitrilvalsartan-figure5.jpg - sacubitrilvalsartan figure5

This is a description of a medication called Sacubitril and Valsartan Tablets. Each film-coated tablet consists of 24 mg sacubitril and 26 mg valsartan. The usual dosage instructions can be found in the prescribing information. The tablets should be stored at 20° to 25°C (68° to 77°F) with excursions permitted between 15° to 30°C (59° to 86°F) to protect from moisture. The packaging includes 30 tablets and has a drug code NDC 42385-930-30. The manufacturer is listed as Laurus Generics Inc., located at 400 Connell Drive, Suite 5200, Berkeley Heights, NJ 07922.*
sacubitrilvalsartan-figure6.jpg - sacubitrilvalsartan figure6

This text provides detailed information about sacubitril and valsartan tablets containing 24 mg sacubitril and 26 mg valsartan. It mentions that the tablets are for institutional use only and come in a package of 100 unit-dose tablets. The usual dosage instructions are not provided, but it instructs to refer to the prescribing information. Storage conditions are specified to be between 20° to 25°C (68° to 77°F) with excursions permitted between 15° to 30°C (59° to 86°F). The product should be protected from moisture and kept away from children. The manufacturing details are given for Laurus Generics Inc. in the USA and Laurus Labs in India.*
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 24 mg/26 mg - cold form Blister (1 x 10's count) - sacubitrilvalsartan figure7

The text provides information about a medication called Sacubitril and Valsartan Tablet with a dosage of 24 mg/26 mg. The medication is manufactured by Laurus Labs Limited. Details such as Lot number and expiration date are also included.*
sacubitrilvalsartan-figure9.jpg - sacubitrilvalsartan figure9
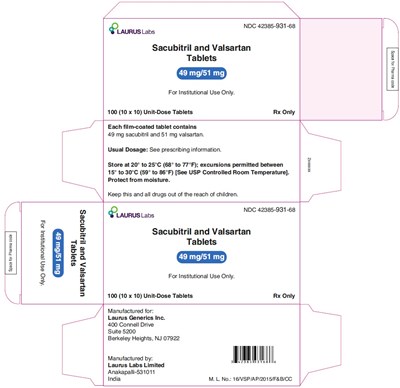
This is information about Sacubitril and Valsartan tablets produced by LAURUS Labs. The tablets contain 49 mg of sacubitril and 51 mg of valsartan each, and are intended for institutional use only. The packaging includes 100 unit-dose tablets (10 x 10). The usual dosage should be followed as per the prescribing information. The storage temperature should be maintained between 20° to 25°C (68° to 77°F) with excursions allowed between 15° to 30°C (59° to 86°F). It is advised to protect the tablets from moisture and keep them out of reach of children. The manufacturing details include Laurus Generics Inc. in New Jersey, USA, and Laurus Labs Limited in India.*
sacubitrilvalsartan-structure - sacubitrilvalsartan structure

This text is not sufficient for generating a description.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.