Product Images Atorvastatin Calcium
View Photos of Packaging, Labels & Appearance
- figure1 - atorvastatin figure1
- figure10 - atorvastatin figure10
- figure11 - atorvastatin figure11
- figure12 - atorvastatin figure12
- figure13 - atorvastatin figure13
- figure14 - atorvastatin figure14
- figure15 - atorvastatin figure15
- figure16 - atorvastatin figure16
- figure17 - atorvastatin figure17
- figure2 - atorvastatin figure2
- figure3 - atorvastatin figure3
- figure4 - atorvastatin figure4
- figure5 - atorvastatin figure5
- figure6 - atorvastatin figure6
- figure7 - atorvastatin figure7
- figure8 - atorvastatin figure8
- figure9 - atorvastatin figure9
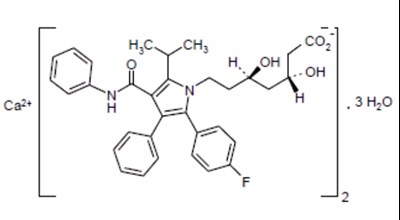
- atorvastatin-structure - atorvastatin structure
Product Label Images
The following 18 images provide visual information about the product associated with Atorvastatin Calcium NDC 42385-941 by Laurus Labs Limited, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
figure1 - atorvastatin figure1

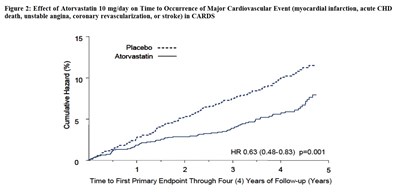
This text provides information about the effect of Atorvastatin 10 mg/day on the cumulative incidence of non-fatal myocardial infarction or coronary heart disease death in a study referred to as ASCOT-LLA. Figure 1 shows a graph depicting the cumulative incidence (%) over a period of 3.0 to 3.5 years. The graph compares the outcomes of patients treated with Atorvastatin (represented by the label "Atorvastatin") versus those who received a placebo (represented by the label "Placebo"). The graph suggests that Atorvastatin may have a beneficial effect in reducing the cumulative incidence of these cardiovascular events. The hazard ratio (HR) for Atorvastatin compared to placebo is reported as 0.64 with a 95% confidence interval from 0.50 to 0.83, and a p-value of 0.0005.*
figure10 - atorvastatin figure10

Each tablet contains 40 mg of atorvastatin calcium anhydrous. The usual dosage can be found in the package insert. The tablets should be stored between 20° to 25°C (68° to 77°F), with excursions permitted between 15° to 30°C (59° to 86°F). They should be dispensed in tight containers. The tablets are manufactured by Laurus Generics Inc. The manufacturing site is located at 400 Connell Drive, Suite 5200, Berkeley Heights, NJ 07922. The tablets are manufactured by Laurus Labs Limited in Anakapalli-531011.*
figure13 - atorvastatin figure13

This text provides information about a medication called Atorvastatin. Each tablet contains 80 mg of atorvastatin calcium anhydrous. The usual dosage should be determined by consulting the package insert for full prescribing information. The tablets should be stored at a temperature between 20°C to 25°C (68°F to 77°F), with excursions permitted between 15°C to 30°C (59°F to 86°F). The medication should be dispensed in tight containers. The label includes a manufacturer name, Laurus Generics Inc., with an address in Berkeley Heights, NJ. The tablets are distributed by LAURUS Labs and the product is intended for prescription use only.*
figure4 - atorvastatin figure4

This text is a description of a medication called Atorvastatin Calcium Tablets. Each tablet contains 10 mg of atorvastatin. The usual dosage and prescribing information can be found in the package insert. The tablets should be stored at temperatures between 20°C to 25°C (68°F to 77°F) with excursions permitted between 15°C to 30°C (59°F to 86°F). The medication should be dispensed in tight containers. The text also includes manufacturing information for Laurus Generics Inc. and Laurus Labs Limited.*
figure7 - atorvastatin figure7

Description: This text provides information about a medication called Atorvastatin. Each tablet contains 20 mg of Atorvastatin, specifically Atorvastatin calcium anhydrous USP. The usual dosage can be found in the package insert. The tablets should be stored between 20°C to 25°C (68°F to 77°F), with excursions permitted between 15°C to 30°C (59°F to 86°F). The medication should be dispensed in tight containers. The text also includes manufacturing information, indicating that the tablets are manufactured for Laurus Generics Inc., located at 400 Connell Drive, Suite 5200, Berkeley Heights, NJ 07922. The tablets are manufactured by Laurus Labs Limited, located in Anakapalli-531011. The text also includes reference numbers and codes related to the medication.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.