FDA Label for Temazepam
View Indications, Usage & Precautions
- WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; AND DEPENDENCE AND WITHDRAWAL REACTIONS
- DESCRIPTION
- PHARMACOKINETICS
- BIOAVAILABILITY, INDUCTION, AND PLASMA LEVELS
- ELIMINATION RATE OF BENZODIAZEPINE HYPNOTICS AND PROFILE OF COMMON UNTOWARD EFFECTS
- CONTROLLED TRIALS SUPPORTING EFFICACY
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- RISKS FROM CONCOMITANT USE WITH OPIOIDS
- ABUSE, MISUSE, AND ADDICTION
- DEPENDENCE AND WITHDRAWAL REACTIONS
- SEVERE ANAPHYLACTIC AND ANAPHYLACTOID REACTIONS
- GENERAL
- INFORMATION FOR PATIENTS
- “SLEEP-DRIVING” AND OTHER COMPLEX BEHAVIORS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- ADVERSE REACTIONS
- CONTROLLED SUBSTANCE
- ABUSE
- DEPENDENCE
- OVERDOSAGE
- TREATMENT
- DOSAGE AND ADMINISTRATION
- DISCONTINUATION OR DOSAGE REDUCTION OF TEMAZEPAM CAPSULES
- HOW SUPPLIED
- STORAGE AND HANDLING
- MEDICATION GUIDE
Temazepam Product Label
The following document was submitted to the FDA by the labeler of this product Solco Healthcare U.s., Llc. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Warning: Risks From Concomitant Use With Opioids; Abuse, Misuse, And Addiction; And Dependence And Withdrawal Reactions
• Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation (see WARNINGS and PRECAUTIONS).• The use of benzodiazepines, including temazepam, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing temazepam capsules and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (see WARNINGS).• The continued use of benzodiazepines, including temazepam, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of temazepam capsules after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue temazepam capsules or reduce the dosage (see DOSAGE AND ADMINISTRATION and WARNINGS).
Description
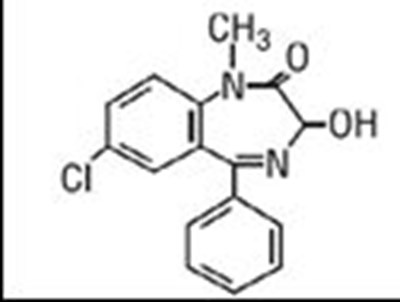
Temazepam capsules USP are benzodiazepine hypnotic agents. The chemical name is 7-chloro-1, 3-dihydro-3-hydroxy-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one, and the structural formula is:
C16H13ClN2O2 MW = 300.74
Temazepam is a white, crystalline substance, very slightly soluble in water and sparingly soluble in alcohol USP.
Temazepam Capsules USP, 7.5 mg, 15 mg, 22.5 mg, and 30 mg, are for oral administration.
7.5 mg, 15 mg, 22.5 mg, and 30 mg Capsules
Active Ingredient: temazepam, USP
Each capsule contains the following inactive ingredients: colloidal silicon dioxide, edetate disodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch and sodium lauryl sulfate. The capsule shells for all strengths contain FD&C Blue #1, FD&C Red #40, gelatin and titanium dioxide. The imprinting ink may contain butyl alcohol, D&C Yellow #10 Aluminum Lake, FD&C Blue #1 Aluminum Lake, FD&C Blue #2 Aluminum Lake, FD&C Red #40 Aluminum Lake, black iron oxide, propylene glycol, n-butyl alcohol and shellac glaze.
Pharmacokinetics
In a single and multiple dose absorption, distribution, metabolism, and excretion (ADME) study, using 3H labeled drug, temazepam was well absorbed and found to have minimal (8%) first pass metabolism. There were no active metabolites formed and the only significant metabolite present in blood was the O-conjugate. The unchanged drug was 96% bound to plasma proteins. The blood level decline of the parent drug was biphasic with the short half-life ranging from 0.4 to 0.6 hours and the terminal half-life from 3.5 to 18.4 hours (mean 8.8 hours), depending on the study population and method of determination. Metabolites were formed with a half-life of 10 hours and excreted with a half-life of approximately 2 hours. Thus, formation of the major metabolite is the rate limiting step in the biodisposition of temazepam. There is no accumulation of metabolites. A dose-proportional relationship has been established for the area under the plasma concentration/time curve over the 15 to 30 mg dose range.
Temazepam was completely metabolized through conjugation prior to excretion; 80% to 90% of the dose appeared in the urine. The major metabolite was the O-conjugate of temazepam (90%); the O-conjugate of N-desmethyl temazepam was a minor metabolite (7%).
Bioavailability, Induction, And Plasma Levels
Following ingestion of a 30 mg temazepam capsule, measurable plasma concentrations were achieved 10 to 20 minutes after dosing with peak plasma levels ranging from 666 to 982 ng/mL (mean 865 ng/mL) occurring approximately 1.2 to 1.6 hours (mean 1.5 hours) after dosing.
In a 7 day study, in which subjects were given a 30 mg temazepam capsule 1 hour before retiring, steady-state (as measured by the attainment of maximal trough concentrations) was achieved by the third dose. Mean plasma levels of temazepam (for days 2 to 7) were 260±210 ng/mL at 9 hours and 75±80 ng/mL at 24 hours after dosing. A slight trend toward declining 24 hour plasma levels was seen after day 4 in the study, however, the 24 hour plasma levels were quite variable.
At a dose of 30 mg once-a-day for 8 weeks, no evidence of enzyme induction was found in man.
Elimination Rate Of Benzodiazepine Hypnotics And Profile Of Common Untoward Effects
The type and duration of hypnotic effects and the profile of unwanted effects during administration of benzodiazepine hypnotics may be influenced by the biologic half-life of the administered drug and for some hypnotics, the half-life of any active metabolites formed. Benzodiazepine hypnotics have a spectrum of half-lives from short (<4 hours) to long (>20 hours). When half-lives are long, drug (and for some drugs their active metabolites) may accumulate during periods of nightly administration and be associated with impairments of cognitive and/or motor performance during waking hours; the possibility of interaction with other psychoactive drugs or alcohol will be enhanced. In contrast, if half-lives are shorter, drug (and, where appropriate, its active metabolites) will be cleared before the next dose is ingested, and carry-over effects related to excessive sedation or CNS depression should be minimal or absent. However, during nightly use for an extended period, pharmacodynamic tolerance or adaptation to some effects of benzodiazepine hypnotics may develop. If the drug has a short elimination half-life, it is possible that a relative deficiency of the drug, or, if appropriate, its active metabolites (i.e., in relationship to the receptor site) may occur at some point in the interval between each night’s use. This sequence of events may account for 2 clinical findings reported to occur after several weeks of nightly use of rapidly eliminated benzodiazepine hypnotics, namely, increased wakefulness during the last third of the night, and the appearance of increased signs of daytime anxiety.
Controlled Trials Supporting Efficacy
Temazepam improved sleep parameters in clinical studies. Residual medication effects (“hangover”) were essentially absent. Early morning awakening, a particular problem in the geriatric patient, was significantly reduced.
Patients with chronic insomnia were evaluated in 2 week, placebo controlled sleep laboratory studies with temazepam at doses of 7.5 mg, 15 mg, and 30 mg, given 30 minutes prior to bedtime. There was a linear dose-response improvement in total sleep time and sleep latency, with significant drug-placebo differences at 2 weeks occurring only for total sleep time at the 2 higher doses, and for sleep latency only at the highest dose.
In these sleep laboratory studies, REM sleep was essentially unchanged and slow wave sleep was decreased. No measurable effects on daytime alertness or performance occurred following temazepam treatment or during the withdrawal period, even though a transient sleep disturbance in some sleep parameters was observed following withdrawal of the higher doses. There was no evidence of tolerance development in the sleep laboratory parameters when patients were given temazepam nightly for at least 2 weeks.
In addition, normal subjects with transient insomnia associated with first night adaptation to the sleep laboratory were evaluated in 24 hour, placebo controlled sleep laboratory studies with temazepam at doses of 7.5 mg, 15 mg, and 30 mg, given 30 minutes prior to bedtime. There was a linear dose-response improvement in total sleep time, sleep latency and number of awakenings, with significant drug-placebo differences occurring for sleep latency at all doses, for total sleep time at the 2 higher doses and for number of awakenings only at the 30 mg dose.
Indications And Usage
Temazepam is indicated for the short-term treatment of insomnia (generally 7 to 10 days).
For patients with short-term insomnia, instructions in the prescription should indicate that Temazepam should be used for short periods of time (7 to 10 days).
The clinical trials performed in support of efficacy were 2 weeks in duration with the final formal assessment of sleep latency performed at the end of treatment.
Contraindications
Benzodiazepines may cause fetal harm when administered to a pregnant woman. An increased risk of congenital malformations associated with the use of diazepam and chlordiazepoxide during the first trimester of pregnancy has been suggested in several studies. Transplacental distribution has resulted in neonatal CNS depression following the ingestion of therapeutic doses of a benzodiazepine hypnotic during the last weeks of pregnancy.
Reproduction studies in animals with temazepam were performed in rats and rabbits. In a perinatal-postnatal study in rats, oral doses of 60 mg/kg/day resulted in increasing nursling mortality. Teratology studies in rats demonstrated increased fetal resorptions at doses of 30 and 120 mg/kg in one study and increased occurrence of rudimentary ribs, which are considered skeletal variants, in a second study at doses of 240 mg/kg or higher. In rabbits, occasional abnormalities such as exencephaly and fusion or asymmetry of ribs were reported without dose relationship. Although these abnormalities were not found in the concurrent control group, they have been reported to occur randomly in historical controls. At doses of 40 mg/kg or higher, there was an increased incidence of the 13th rib variant when compared to the incidence in concurrent and historical controls.
Temazepam is contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Patients should be instructed to discontinue the drug prior to becoming pregnant. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered.
Risks From Concomitant Use With Opioids
Concomitant use of benzodiazepines, including temazepam, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe temazepam concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of temazepam than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking temazepam, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when temazepam is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see PRECAUTIONS, Drug Interactions).
Abuse, Misuse, And Addiction
The use of benzodiazepines, including temazepam capsules, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see DRUG ABUSE AND DEPENDENCE, Abuse).
Before prescribing temazepam capsules and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of temazepam capsules, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of temazepam capsules along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence And Withdrawal Reactions
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue temazepam capsules or reduce the dosage (a patient-specific plan should be used to taper the dose) (see DOSAGE AND ADMINISTRATION, Discontinuation or Dosage Reduction of Temazepam capsules).
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal Reactions
The continued use of benzodiazepines, including temazepam capsules, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of temazepam capsules after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see DRUG ABUSE AND DEPENDENCE, Dependence).
Protracted Withdrawal Syndrome
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see DRUG ABUSE AND DEPENDENCE, Dependence).
Sleep disturbance may be the presenting manifestation of an underlying physical and/or psychiatric disorder. Consequently, a decision to initiate symptomatic treatment of insomnia should only be made after the patient has been carefully evaluated. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia may be the consequence of an unrecognized psychiatric or physical disorder as may the emergence of new abnormalities of thinking or behavior. Such abnormalities have also been reported to occur in association with the use of drugs with central nervous system depressant activity, including those of the benzodiazepine class. Because some of the worrisome adverse effects of benzodiazepines, including temazepam, appear to be dose related (see PRECAUTIONS and DOSAGE AND ADMINISTRATION), it is important to use the lowest possible effective dose. Elderly patients are especially at risk.
Some of these changes may be characterized by decreased inhibition, e.g., aggressiveness and extroversion that seem out of character, similar to that seen with alcohol. Other kinds of behavioral changes can also occur, for example, bizarre behavior, agitation, hallucinations, and depersonalization. Complex behaviors such as “sleep-driving” (i.e., driving while not fully awake after ingestion of a sedative-hypnotic, with amnesia for the event) have been reported. These events can occur in sedative-hypnotic-naïve as well as in sedative-hypnotic-experienced persons. Although behaviors such as sleep-driving may occur with temazepam alone at therapeutic doses, the use of alcohol and other CNS depressants with temazepam appears to increase the risk of such behaviors, as does the use of temazepam at doses exceeding the maximum recommended dose. Due to the risk to the patient and the community, discontinuation of temazepam should be strongly considered for patients who report a “sleep-driving” episode. Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events. Amnesia and other neuro-psychiatric symptoms may occur unpredictably. In primarily depressed patients, worsening of depression, including suicidal thinking has been reported in association with the use of sedative/hypnotics.
It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
Because temazepam can cause drowsiness and a decreased level of consciousness, patients, particularly the elderly, are at higher risk of falls.
Severe Anaphylactic And Anaphylactoid Reactions
Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including temazepam. Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis. Some patients have required medical therapy in the emergency department. If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with temazepam should not be rechallenged with the drug.
General
Since the risk of the development of oversedation, dizziness, confusion, and/or ataxia increases substantially with larger doses of benzodiazepines in elderly and debilitated patients, 7.5 mg of temazepam is recommended as the initial dosage for such patients.
Temazepam should be administered with caution in severely depressed patients or those in whom there is any evidence of latent depression; it should be recognized that suicidal tendencies may be present and protective measures may be necessary.
The usual precautions should be observed in patients with impaired renal or hepatic function and in patients with chronic pulmonary insufficiency.
If temazepam is to be combined with other drugs having known hypnotic properties or CNS- depressant effects, consideration should be given to potential additive effects.
The possibility of a synergistic effect exists with the co-administration of temazepam and diphenhydramine. One case of stillbirth at term has been reported 8 hours after a pregnant patient received temazepam and diphenhydramine. A cause and effect relationship has not yet been determined (see CONTRAINDICATIONS).
Information For Patients
Advise the patient to read the FDA approved patient labeling (Medication Guide).
Risks from Concomitant Use with Opioids
Advise both patients and caregivers about the risks of potentially fatal respiratory depression and sedation when temazepam capsules is used with opioids and not to use such drugs concomitantly unless supervised by a healthcare provider. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see WARNINGS, Risks from Concomitant Use with Opioidsand PRECAUTIONS, Drug Interactions).
Abuse, Misuse, and Addiction
Inform patients that the use of temazepam capsules, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug (see WARNINGS, Abuse, Misuse, and Addictionand DRUG ABUSE AND DEPENDENCE).
Withdrawal Reactions
Inform patients that the continued use of temazepam capsules may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of temazepam capsules may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months. Instruct patients that discontinuation or dosage reduction of temazepam capsules may require a slow taper (see WARNINGS, Dependence and Withdrawal Reactionsand DRUG ABUSE AND DEPENDENCE).
“Sleep-Driving” And Other Complex Behaviors
There have been reports of people getting out of bed after taking a sedative-hypnotic and driving their cars while not fully awake, often with no memory of the event. If a patient experiences such an episode, it should be reported to his or her doctor immediately, since “sleep-driving” can be dangerous. This behavior is more likely to occur when temazepam is taken with alcohol or other central nervous system depressants (see WARNINGS). Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events.
Advise patients that increased drowsiness and decreased consciousness may increase the risk of falls in some patients.
Laboratory Tests
The usual precautions should be observed in patients with impaired renal or hepatic function and in patients with chronic pulmonary insufficiency. Abnormal liver function tests as well as blood dyscrasias have been reported with benzodiazepines.
Drug Interactions
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists.
Limit dosage and duration of concomitant use of benzodiazepines and opioids, and monitor patients closely for respiratory depression and sedation.
The pharmacokinetic profile of temazepam does not appear to be altered by orally administered cimetidine dosed according to labeling.
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenicity studies were conducted in rats at dietary temazepam doses up to 160 mg/kg/day for 24 months and in mice at dietary doses of 160 mg/kg/day for 18 months. No evidence of carcinogenicity was observed although hyperplastic liver nodules were observed in female mice exposed to the highest dose. The clinical significance of this finding is not known.
Fertility in male and female rats was not adversely affected by temazepam.
No mutagenicity tests have been done with temazepam.
Pregnancy
Pregnancy Category X (see CONTRAINDICATIONS).
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when temazepam is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Clinical studies of temazepam did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy commonly observed in this population. Temazepam 7.5 mg is recommended as the initial dosage for patients aged 65 and over since the risk of the development of oversedation, dizziness, confusion, ataxia and/or falls increases substantially with larger doses of benzodiazepines in elderly and debilitated patients.
Adverse Reactions
During controlled clinical studies in which 1076 patients received temazepam at bedtime, the drug was well tolerated. Side effects were usually mild and transient. Adverse reactions occurring in 1% or more of patients are presented in the following table:
| Temazepam | Placebo |
Drowsiness | 9.1 | 5.6 |
Headache | 8.5 | 9.1 |
Fatigue | 4.8 | 4.7 |
Nervousness | 4.6 | 8.2 |
Lethargy | 4.5 | 3.4 |
Dizziness | 4.5 | 3.3 |
Nausea | 3.1 | 3.8 |
Hangover | 2.5 | 1.1 |
Anxiety | 2.0 | 1.5 |
Depression | 1.7 | 1.8 |
Dry Mouth | 1.7 | 2.2 |
Diarrhea | 1.7 | 1.1 |
Abdominal Discomfort | 1.5 | 1.9 |
Euphoria | 1.5 | 0.4 |
Weakness | 1.4 | 0.9 |
Confusion | 1.3 | 0.5 |
Blurred Vision | 1.3 | 1.3 |
Nightmares | 1.2 | 1.7 |
Vertigo | 1.2 | 0.8 |
The following adverse events have been reported less frequently (0.5% to 0.9%):
Central Nervous System – anorexia, ataxia, equilibrium loss, tremor, increased dreaming
Cardiovascular – dyspnea, palpitations
Gastrointestinal – vomiting
Musculoskeletal – backache
Special Senses – hyperhidrosis, burning eyes
Amnesia, hallucinations, horizontal nystagmus, and paradoxical reactions including restlessness, overstimulation and agitation were rare (less than 0.5%).
To report SUSPECTED ADVERSE REACTIONS, contact Solco Healthcare US, LLC at 1-866-257-2597 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Controlled Substance
Temazepam capsules contains temazepam, a Schedule IV controlled substance.
Abuse
Temazepam capsules is a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a healthcare provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence. Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medication. Abuse and misuse of benzodiazepines may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders (see WARNINGS, Abuse, Misuse, and Addiction).
The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia, suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
Dependence
Physical Dependence
Temazepam capsules may produce physical dependence from continued therapy. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Abrupt discontinuation or rapid dosage reduction of benzodiazepines or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use (seeWARNINGS, Dependence and Withdrawal Reactions).
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue temazepam capsules or reduce the dosage (see DOSAGE and ADMINISTRATION, Discontinuation or Dosage Reduction of Temazepam capsulesandWARNINGS, Dependence and Withdrawal Reactions).
Tolerance
Tolerance to temazepam capsules may develop from continued therapy. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to the therapeutic effect of temazepam capsules may develop; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines.
Overdosage
Manifestations of acute overdosage of temazepam can be expected to reflect the CNS effects of the drug and include somnolence, confusion, and coma, with reduced or absent reflexes, respiratory depression, and hypotension. The oral LD50 of temazepam was 1963 mg/kg in mice, 1833 mg/kg in rats, and >2400 mg/kg in rabbits.
Treatment
If the patient is conscious, vomiting should be induced mechanically or with emetics. Gastric lavage should be employed utilizing concurrently a cuffed endotracheal tube if the patient is unconscious to prevent aspiration and pulmonary complications. Maintenance of adequate pulmonary ventilation is essential. The use of pressor agents intravenously may be necessary to combat hypotension. Fluids should be administered intravenously to encourage diuresis. The value of dialysis has not been determined. If excitation occurs, barbiturates should not be used. It should be borne in mind that multiple agents may have been ingested. Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation, and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for re-sedation, respiratory depression, and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert including CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS should be consulted prior to use.
Up-to-date information about the treatment of overdose can be obtained from a certified Regional Poison Control Center.
Dosage And Administration
While the recommended usual adult dose is 15 mg before retiring, 7.5 mg may be sufficient for some patients, and others may need 30 mg. In transient insomnia, a 7.5 mg dose may be sufficient to improve sleep latency. In elderly or debilitated patients, it is recommended that therapy be initiated with 7.5 mg until individual responses are determined.
Discontinuation Or Dosage Reduction Of Temazepam Capsules
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue temazepam capsules or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly (see WARNINGS, Dependence and Withdrawal Reactions and DRUG ABUSE AND DEPENDENCE: Dependence).
How Supplied
Temazepam Capsules USP
7.5 mg
Opaque Blue and Opaque pink capsules, with the opaque pink body imprinted “7.5mg” in black, and opaque blue cap imprinted “S611” in black. The capsules are supplied in bottles of 100 (43547-611-10).
15 mg
Opaque Scarlet and Opaque pink capsules, with the Opaque pink body imprinted “15mg” in black, and Opaque Scarlet cap imprinted “S612” in black. The capsules are supplied in bottles of 100 (43547-612-10) and 500 (43547-612-50).
22.5 mg
Opaque Blue capsules, with the Opaque Blue body imprinted “22.5mg” in black, and Opaque Blue cap imprinted “S613” in black. The capsules are supplied in bottles of 30 (43547-613-03).
30 mg
Opaque Scarlet and Opaque blue capsules, with the opaque blue body imprinted “30mg” in black, and Opaque scarlet cap imprinted “S614” in black. The capsules are supplied in bottles of 100 (43547-614-10) and 500 (43547-614-50).
Storage And Handling
Dispense in a well-closed, light-resistant container with a child-resistant closure.
Storage: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Medication Guide
Temazepam Capsules, USP
(təˈmæzɪpæm)
What is the most important information I should know about temazepam capsules?
• Temazepam capsules is a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system (CNS) depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma and death. Get emergency help right away if any of the following happens:o shallow or slowed breathingo breathing stops (which may lead to the heart stopping)o excessive sleepiness (sedation)o Risk of abuse, misuse, and addiction. There is a risk of abuse, misuse, and addiction with benzodiazepines including temazepam capsules which can lead to overdose and serious side effects including coma and death. Serious side effects including coma and death have happened in people who have abused or misused benzodiazepines, including temazepam capsules. These serious side effects may also include delirium, paranoia, suicidal thoughts or actions, seizures, and difficulty breathing. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these serious side effects.o You can develop an addiction even if you take temazepam capsules as prescribed by your healthcare provider.o Take temazepam capsules exactly as your healthcare provider prescribed.o Do not share your temazepam capsules with other people.o Keep temazepam capsules in a safe place and away from children.• Physical dependence and withdrawal reactions. Temazepam capsules can cause physical dependence and withdrawal reactions.o Do not suddenly stop taking temazepam capsules. Stopping temazepam capsules suddenly can cause serious and life-threatening side effects, including, unusual movements, responses, or expressions, seizures, sudden and severe mental or nervous system changes, depression, seeing or hearing things that others do not see or hear, an extreme increase in activity or talking, losing touch with reality, and suicidal thoughts or actions. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these symptoms.o Some people who suddenly stop benzodiazepines have symptoms that can last for several weeks to more than 12 months, including, anxiety, trouble remembering, learning, or concentrating, depression, problems sleeping, feeling like insects are crawling under your skin, weakness, shaking, muscle twitching, burning or prickling feeling in your hands, arms, legs or feet, and ringing in your ears.o Physical dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical dependence and drug addiction.o Do not take more temazepam capsules than prescribed or take temazepam capsules for longer than prescribed.• After taking temazepam capsules, you may get up out of bed while not being fully awake and do an activity that you do not know you are doing. The next morning, you may not remember that you did anything during the night. You have a higher chance for doing these activities if you drink alcohol or take other medicines that make you sleepy with temazepam capsules. Reported activities include:o driving a car (“sleep-driving”)o making and eating foodo talking on the phoneo having sexo sleep-walking• Temazepam capsules are a prescription sleep medicine. Temazepam capsules are used in adults for the short-term (usually 7 to 10 days) treatment of a sleep problem called insomnia. Symptoms of insomnia include trouble falling asleep and waking up often during the night.• Temazepam capsules are a federal controlled substance (C-IV) because it contains temazepam that can be abused or lead to dependence. Keep temazepam capsules in a safe place to prevent misuse and abuse. Selling or giving away temazepam capsules may harm others, and is against the law. Tell your healthcare provider if you have ever abused or been dependent on alcohol, prescription medicines or street drugs.• It is not known if temazepam capsules safe and effective in children.• It is not known if temazepam capsules safe and effective for use longer than 2 weeks.
Do not drive or operate heavy machinery until you know how taking temazepam capsules and opioids affects you.
Call your healthcare provider right away if you find out that you have done any of the above activities after taking temazepam capsules.
What are temazepam capsules?
* Please review the disclaimer below.