Product Images Rivaroxaban
View Photos of Packaging, Labels & Appearance
- 30 tablets - rivaroxaban 10
- 51 tablets - rivaroxaban 15 20
- 30 tablets - rivaroxaban 15
- 60 tablets - rivaroxaban 2 5
- 30 tablets - rivaroxaban 20
- rivaroxaban-fig-1 - rivaroxaban fig 1
- rivaroxaban-fig-2.jpg - rivaroxaban fig 2
- rivaroxaban-fig-3.jpg - rivaroxaban fig 3
- rivaroxaban-fig5.jpg - rivaroxaban fig5
- rivaroxaban-figure10.jpg - rivaroxaban figure10
- rivaroxaban-figure11.jpg - rivaroxaban figure11
- rivaroxaban-figure12.jpg - rivaroxaban figure12
- rivaroxaban-figure4.jpg - rivaroxaban figure4
- rivaroxaban-figure6.jpg - rivaroxaban figure6
- rivaroxaban-figure7.jpg - rivaroxaban figure7
- rivaroxaban-figure8.jpg - rivaroxaban figure8
- rivaroxaban-figure9.jpg - rivaroxaban figure9
- rivaroxaban-str - rivaroxaban structure
Product Label Images
The following 18 images provide visual information about the product associated with Rivaroxaban NDC 46708-683 by Alembic Pharmaceuticals Limited, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
60 tablets - rivaroxaban 2 5

This text appears to be the label information for a medication named Rivaroxaban, in tablet form with a strength of 25mg. The recommended temperature for storage is within the range of 15-30°C (55-86°F). The package contains 60 tablets and a Medication Guide for patients. The manufacturer's contact information is provided at the bottom. The National Drug Code (NDC) for this product is 46708-683-60. It is important to keep the medication in a tightly closed container and out of reach of children. Be sure to follow the full prescribing information for this medication.*
rivaroxaban-fig-2.jpg - rivaroxaban fig 2
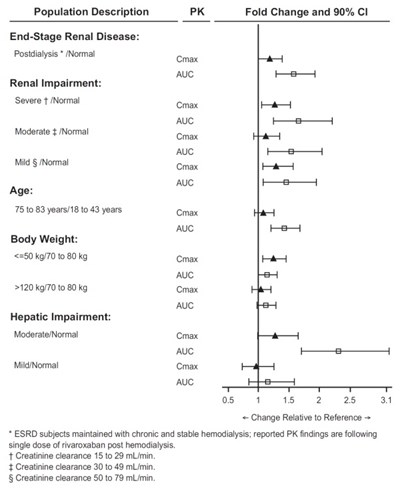
Population Description PK Fold Change and 90% CI across different health conditions and demographics such as End-Stage Renal Disease, Renal Impairment, Age, Body Weight, and Hepatic Impairment. The data includes comparisons of various parameters like AUC, Cmax, and creatinine clearance in different subgroups. The findings are based on single doses of rivaroxaban post hemodialysis for ESRD subjects and provide insights into how these factors may influence pharmacokinetics.*
rivaroxaban-fig-3.jpg - rivaroxaban fig 3
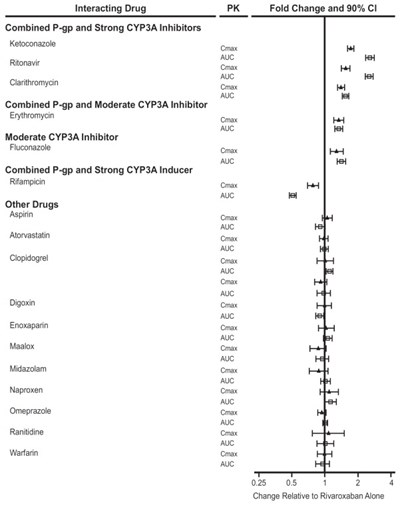
This text provides information on the effect of different drug interactions on the pharmacokinetics (PK) of specific drugs, including changes in area under the curve (AUC) and maximum concentration (Cmax) values. The drugs mentioned include inhibitors and inducers of P-glycoprotein (P-gp) and CYP3A enzymes, as well as other common medications like aspirin, atorvastatin, and clopidogrel. The data presented in the text shows the fold change and 90% confidence interval (CI) for each drug interaction compared to the PK parameters of Rivaroxaban alone.*
rivaroxaban-figure11.jpg - rivaroxaban figure11

This text seems to be a table showing the number of subjects at risk over a certain period in a study comparing Rivaroxaban and Placebo treatments. It includes data on the number of tablets, days from randomization, and the respective number of subjects at risk for each treatment group. The data suggests a clinical trial scenario where the subjects are monitored over time to evaluate the effectiveness and safety of the two treatments.*
rivaroxaban-figure4.jpg - rivaroxaban figure4

This text provides information on the Cumulative Event Rate (%) for Rivaroxaban Tablets and Warfarin over a specified period of time. It also includes the Hazard Ratio (HR) with its corresponding 95% Confidence Interval (CI). The data seems to be related to a clinical study comparing the event rates between the two treatments. The days from randomization are also mentioned, possibly indicating the timeline of the study.*
rivaroxaban-figure6.jpg - rivaroxaban figure6

This text provides information on the cumulative event rate (%) for Enoxaparin/VKA and Rivaroxaban tablets. It includes a hazard ratio (HR) with a 95% confidence interval of 0.68 (0.44, 1.04) and denotes the timeline in days from randomization.*
rivaroxaban-figure7.jpg - rivaroxaban figure7

This is data related to the Cumulative Event Rate (%) over time for the drugs Enoxaparin and VKA compared to Rivaroxaban Tablets. The graph shows the event rate over a period of 360 days from randomization, with the Hazard Ratio (HR) and its 95% Confidence Interval (CI) indicated at day 270.*
rivaroxaban-figure8.jpg - rivaroxaban figure8

This text seems to be a table showing the cumulative event rate and hazard ratio of Rivaroxaban Tablets in comparison to Aspirin over a period of time. It provides information on the number of subjects at risk for both treatments. The hazard ratio with a 95% confidence interval is also listed in the table.*
rivaroxaban-figure9.jpg - rivaroxaban figure9

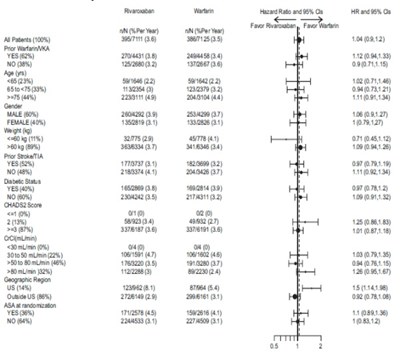
This data appears to be a comparison between the efficacy of Rivaroxaban tablets and placebo in preventing outcomes such as stroke, major cardiovascular events, and death. It includes information on different age groups, sex, region, weight, kidney function, medical history, and other factors to evaluate the effectiveness of Rivaroxaban tablets. The results suggest that Rivaroxaban tablets may have benefits compared to placebo in some of the mentioned categories.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.