FDA Label for Hand Sanitizer
View Indications, Usage & Precautions
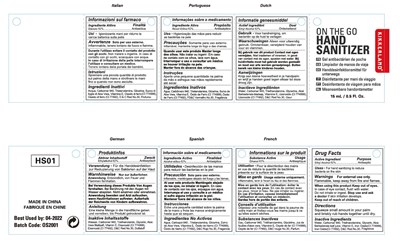
Hand Sanitizer Product Label
The following document was submitted to the FDA by the labeler of this product Ningbo Jiangbei Ocean Star Trading Co.,ltd. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Otc - Active Ingredient
Active ingredients
Ethyl Alcohol 62%
Otc - Purpose
Antiseptic
Indications & Usage
Uses:
For hand sanitizing to reduce bacteria on the skin.
Warnings
Warnings:
For external use only.
Flammable. Keep away from fire or flame.
Otc - When Using
When using this product Keep out of reach of eyes. In case of eye contact, flush with water. Do not inhale or ingest.
Otc - Stop Use
Stop use and ask a doctor if skin irritation develops.
Otc - Keep Out Of Reach Of Children
Keep out of reach of children.
Dosage & Administration
Directions:
Squeeze small amount in your palm and briskly rub hands together until dry.
Inactive Ingredient
Inactive Ingredients:
Water,Carbomer 940,Triethanolamine,Glycerin,Aloe Barbadensis leaf juice,Vitamin E,Iron Oxide(CI 774999),Iron Oxide(CI 77492),D&C Red No.30,Fragrance
Package Label.Principal Display Panel
* Please review the disclaimer below.