Product Images Flovent Diskus
View Photos of Packaging, Labels & Appearance
- Chemical structure - flovent diskus spl graphic 01
- Figure 1. A 12-Week Clinical Trial Evaluating FLOVENT DISKUS 100 mcg Twice Daily in Adults and Adolescents Receiving Bronchodilators Alone - flovent diskus spl graphic 02
- Figure 2. A 12-Week Clinical Trial Evaluating FLOVENT DISKUS 100 mcg Twice Daily in Adults and Adolescents Receiving Inhaled Corticosteroids - flovent diskus spl graphic 03
- Figure 3. A 12-Week Clinical Trial Evaluating FLOVENT DISKUS 250 mcg Twice Daily in Adults and Adolescents Receiving Inhaled Corticosteroids or Bronchodilators Alone - flovent diskus spl graphic 04
- Figure 4. A 12-Week Clinical Trial Evaluating FLOVENT DISKUS 500 mcg Twice Daily in Adults and Adolescents Receiving Inhaled Corticosteroids or Bronchodilators Alone - flovent diskus spl graphic 05
- Figure A - flovent diskus spl graphic 06
- Figure B - flovent diskus spl graphic 07
- Figure C - flovent diskus spl graphic 08
- Figure D - flovent diskus spl graphic 09
- Figure E - flovent diskus spl graphic 10
- Figure F - flovent diskus spl graphic 11
- Figure G - flovent diskus spl graphic 12
- Figure H - flovent diskus spl graphic 13
- Label Image - lbl500901245
Product Label Images
The following 14 images provide visual information about the product associated with Flovent Diskus NDC 50090-1245 by A-s Medication Solutions, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1. A 12-Week Clinical Trial Evaluating FLOVENT DISKUS 100 mcg Twice Daily in Adults and Adolescents Receiving Bronchodilators Alone - flovent diskus spl graphic 02
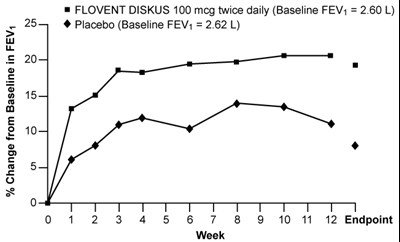
Figure 2. A 12-Week Clinical Trial Evaluating FLOVENT DISKUS 100 mcg Twice Daily in Adults and Adolescents Receiving Inhaled Corticosteroids - flovent diskus spl graphic 03

This text appears to be a comparison between two treatments: FLOVENT DISKUS 100 mcg taken twice daily and a placebo. Baseline FEV (Forced Expiratory Volume) measurements are provided for both treatments. Additionally, there is a table or graph showing the percentage change in FEV over time.*
Figure 3. A 12-Week Clinical Trial Evaluating FLOVENT DISKUS 250 mcg Twice Daily in Adults and Adolescents Receiving Inhaled Corticosteroids or Bronchodilators Alone - flovent diskus spl graphic 04
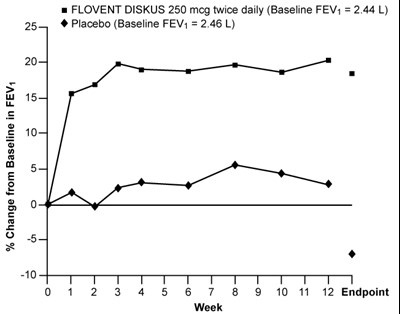
FLOVENT DISKUS 250 mcg is a medication that is taken twice daily. It is used to treat respiratory conditions such as asthma. The baseline FEV1 (forced expiratory volume in one second) of the patient is 2.44 L. There is also a placebo group with a baseline FEV1 of 2.46 L. The % change from baseline in FEV1 is being measured over the course of 12 weeks. It appears that there are multiple data points being collected at various time intervals during the study.*
Figure 4. A 12-Week Clinical Trial Evaluating FLOVENT DISKUS 500 mcg Twice Daily in Adults and Adolescents Receiving Inhaled Corticosteroids or Bronchodilators Alone - flovent diskus spl graphic 05

* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.