FDA Label for Xarelto
View Indications, Usage & Precautions
- OTHER
- 1.1 REDUCTION OF RISK OF STROKE AND SYSTEMIC EMBOLISM IN NONVALVULAR ATRIAL FIBRILLATION
- 1.2 TREATMENT OF DEEP VEIN THROMBOSIS
- 1.3 TREATMENT OF PULMONARY EMBOLISM
- 1.4 REDUCTION IN THE RISK OF RECURRENCE OF DEEP VEIN THROMBOSIS AND/OR PULMONARY EMBOLISM
- 1.5 PROPHYLAXIS OF DEEP VEIN THROMBOSIS FOLLOWING HIP OR KNEE REPLACEMENT SURGERY
- 2.1 RECOMMENDED DOSAGE
- 2.2 IMPORTANT FOOD EFFECT INFORMATION
- 2.4 NONVALVULAR ATRIAL FIBRILLATION
- 2.5 TREATMENT OF DEEP VEIN THROMBOSIS (DVT) AND/OR PULMONARY EMBOLISM (PE)
- 2.6 REDUCTION IN THE RISK OF RECURRENCE OF DEEP VEIN THROMBOSIS (DVT) AND/OR PULMONARY EMBOLISM (PE)
- 2.7 PROPHYLAXIS OF DEEP VEIN THROMBOSIS FOLLOWING HIP OR KNEE REPLACEMENT SURGERY
- 2.8 DISCONTINUATION FOR SURGERY AND OTHER INTERVENTIONS
- 2.9 MISSED DOSE
- 2.10 ADMINISTRATION OPTIONS
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5.1 INCREASED RISK OF THROMBOTIC EVENTS AFTER PREMATURE DISCONTINUATION
- 5.2 RISK OF BLEEDING
- 5.3 SPINAL/EPIDURAL ANESTHESIA OR PUNCTURE
- 5.5 USE IN PATIENTS WITH HEPATIC IMPAIRMENT
- 5.6 USE WITH P-GP AND STRONG CYP3A4 INHIBITORS OR INDUCERS
- 5.7 RISK OF PREGNANCY-RELATED HEMORRHAGE
- 5.8 PATIENTS WITH PROSTHETIC HEART VALVES
- 5.9 ACUTE PE IN HEMODYNAMICALLY UNSTABLE PATIENTS OR PATIENTS WHO REQUIRE THROMBOLYSIS OR PULMONARY EMBOLECTOMY
- 6 ADVERSE REACTIONS
- 6.1 CLINICAL TRIALS EXPERIENCE
- 6.2 POSTMARKETING EXPERIENCE
- 7.1 GENERAL INHIBITION AND INDUCTION PROPERTIES
- 7.3 DRUGS THAT INDUCE CYTOCHROME P450 3A4 ENZYMES AND DRUG TRANSPORT SYSTEMS
- 7.4 ANTICOAGULANTS AND NSAIDS/ASPIRIN
- 8.3 FEMALES AND MALES OF REPRODUCTIVE POTENTIAL
- 8.4 PEDIATRIC USE
- 8.5 GERIATRIC USE
- 8.6 RENAL IMPAIRMENT
- 8.7 HEPATIC IMPAIRMENT
- 10 OVERDOSAGE
- 11 DESCRIPTION
- 12.1 MECHANISM OF ACTION
- 12.2 PHARMACODYNAMICS
- 12.6 QT/QTC PROLONGATION
- 13.1 CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
- 14.1 STROKE PREVENTION IN NONVALVULAR ATRIAL FIBRILLATION
- 14.4 PROPHYLAXIS OF DEEP VEIN THROMBOSIS FOLLOWING HIP OR KNEE REPLACEMENT SURGERY
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- SPL MEDGUIDE
- PRINCIPAL DISPLAY PANEL - 10 MG TABLET BOTTLE LABEL
- PRINCIPAL DISPLAY PANEL - 15 MG TABLET BOTTLE LABEL
- PRINCIPAL DISPLAY PANEL - 20 MG TABLET BOTTLE LABEL
- PRINCIPAL DISPLAY PANEL - KIT CARTON
Xarelto Product Label
The following document was submitted to the FDA by the labeler of this product Janssen Pharmaceuticals, Inc.. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Other
A. Premature discontinuation of XARELTO increases the risk of thrombotic events
Premature discontinuation of any oral anticoagulant, including XARELTO, increases the risk of thrombotic events. If anticoagulation with XARELTO is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant [see Dosage and Administration (2.3, 2.8), Warnings and Precautions (5.1), and Clinical Studies (14.1)].
B. Spinal/epidural hematoma
Epidural or spinal hematomas have occurred in patients treated with XARELTO who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
- use of indwelling epidural catheters
- concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants
- a history of traumatic or repeated epidural or spinal punctures
- a history of spinal deformity or spinal surgery
- optimal timing between the administration of XARELTO and neuraxial procedures is not known
- Advise patients to take XARELTO only as directed.
- Remind patients to not discontinue XARELTO without first talking to their healthcare professional.
- Advise patients with atrial fibrillation to take XARELTO once daily with the evening meal.
- Advise patients for initial treatment of DVT and/or PE to take XARELTO 15 mg or 20 mg tablets with food at approximately the same time every day [see Dosage and Administration (2.5)].
- Advise patients who are at a continued risk of recurrent DVT and/or PE after at least 6 months of initial treatment, to take XARELTO 10 mg once daily with or without food [see Dosage and Administration (2.6)].
- Advise patients who cannot swallow the tablet whole to crush XARELTO and combine with a small amount of applesauce followed by food [see Dosage and Administration (2.10)].
- For patients requiring an NG tube or gastric feeding tube, instruct the patient or caregiver to crush the XARELTO tablet and mix it with a small amount of water before administering via the tube [see Dosage and Administration (2.10)].
- If a dose is missed, advise the patient to take XARELTO as soon as possible on the same day and continue on the following day with their recommended daily dose regimen.
- Advise patients to report any unusual bleeding or bruising to their physician. Inform patients that it might take them longer than usual to stop bleeding, and that they may bruise and/or bleed more easily when they are treated with XARELTO [see Warnings and Precautions (5.2)].
- If patients have had neuraxial anesthesia or spinal puncture, and particularly, if they are taking concomitant NSAIDs or platelet inhibitors, advise patients to watch for signs and symptoms of spinal or epidural hematoma, such as back pain, tingling, numbness (especially in the lower limbs), muscle weakness, and stool or urine incontinence. If any of these symptoms occur, advise the patient to contact his or her physician immediately [see Boxed Warning].
- Advise patients to inform their physician immediately if they become pregnant or intend to become pregnant during treatment with XARELTO [see Use in Specific Populations (8.1)].
- Advise pregnant women receiving XARELTO to immediately report to their physician any bleeding or symptoms of blood loss [see Warnings and Precautions (5.7)].
[see Warnings and Precautions (5.2, 5.3) and Adverse Reactions (6.2)].
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary [see Warnings and Precautions (5.3)].
Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis [see Warnings and Precautions (5.3)].
Switching from Warfarin to XARELTO - When switching patients from warfarin to XARELTO, discontinue warfarin and start XARELTO as soon as the International Normalized Ratio (INR) is below 3.0 to avoid periods of inadequate anticoagulation.
Switching from XARELTO to Warfarin - No clinical trial data are available to guide converting patients from XARELTO to warfarin. XARELTO affects INR, so INR measurements made during coadministration with warfarin may not be useful for determining the appropriate dose of warfarin. One approach is to discontinue XARELTO and begin both a parenteral anticoagulant and warfarin at the time the next dose of XARELTO would have been taken.
Switching from XARELTO to Anticoagulants other than Warfarin - For patients currently taking XARELTO and transitioning to an anticoagulant with rapid onset, discontinue XARELTO and give the first dose of the other anticoagulant (oral or parenteral) at the time that the next XARELTO dose would have been taken [see Drug Interactions (7.4)].
Switching from Anticoagulants other than Warfarin to XARELTO - For patients currently receiving an anticoagulant other than warfarin, start XARELTO 0 to 2 hours prior to the next scheduled evening administration of the drug (e.g., low molecular weight heparin or non-warfarin oral anticoagulant) and omit administration of the other anticoagulant. For unfractionated heparin being administered by continuous infusion, stop the infusion and start XARELTO at the same time.
Administration via nasogastric (NG) tube or gastric feeding tube: After confirming gastric placement of the tube, 10 mg, 15 mg or 20 mg XARELTO tablets may be crushed and suspended in 50 mL of water and administered via an NG tube or gastric feeding tube. Since rivaroxaban absorption is dependent on the site of drug release, avoid administration of XARELTO distal to the stomach which can result in reduced absorption and thereby, reduced drug exposure. After the administration of a crushed XARELTO 15 mg or 20 mg tablet, the dose should then be immediately followed by enteral feeding [see Clinical Pharmacology (12.3)].
Crushed 10 mg, 15 mg or 20 mg XARELTO tablets are stable in water and in applesauce for up to 4 hours. An in vitro compatibility study indicated that there is no adsorption of rivaroxaban from a water suspension of a crushed XARELTO tablet to PVC or silicone nasogastric (NG) tubing.
Reversal of Anticoagulant Effect
A specific antidote for rivaroxaban is not available. Because of high plasma protein binding, rivaroxaban is not expected to be dialyzable [see Clinical Pharmacology (12.3)]. Protamine sulfate and vitamin K are not expected to affect the anticoagulant activity of rivaroxaban. Partial reversal of prothrombin time prolongation has been seen after administration of prothrombin complex concentrates (PCCs) in healthy volunteers. The use of other procoagulant reversal agents like activated prothrombin complex concentrate (APCC) or recombinant factor VIIa (rFVIIa) has not been evaluated.
Nonvalvular Atrial Fibrillation
Periodically assess renal function as clinically indicated (i.e., more frequently in situations in which renal function may decline) and adjust therapy accordingly [see Dosage and Administration (2.4)]. Consider dose adjustment or discontinuation of XARELTO in patients who develop acute renal failure while on XARELTO [see Use in Specific Populations (8.6)].
Treatment of Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), and Reduction in the Risk of Recurrence of DVT and of PE
Avoid the use of XARELTO in patients with CrCl <30 mL/min due to an expected increase in rivaroxaban exposure and pharmacodynamic effects in this patient population [see Use in Specific Populations (8.6)].
Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
Avoid the use of XARELTO in patients with CrCl <30 mL/min due to an expected increase in rivaroxaban exposure and pharmacodynamic effects in this patient population. Observe closely and promptly evaluate any signs or symptoms of blood loss in patients with CrCl 30 to 50 mL/min. Patients who develop acute renal failure while on XARELTO should discontinue the treatment [see Use in Specific Populations (8.6)].
Hemorrhage
The most common adverse reactions with XARELTO were bleeding complications [see Warnings and Precautions (5.2)].
Nonvalvular Atrial Fibrillation
In the ROCKET AF trial, the most frequent adverse reactions associated with permanent drug discontinuation were bleeding events, with incidence rates of 4.3% for XARELTO vs. 3.1% for warfarin. The incidence of discontinuations for non-bleeding adverse events was similar in both treatment groups.
Table 1 shows the number of patients experiencing various types of bleeding events in the ROCKET AF trial.
| Parameter | XARELTO N=7111 n (%/year) | Warfarin N=7125 n (%/year) | XARELTO vs. Warfarin HR (95% CI) |
|---|---|---|---|
| Abbreviations: HR = Hazard Ratio, CI = Confidence interval, CRNM = Clinically Relevant Non-Major. | |||
| Major Bleeding Defined as clinically overt bleeding associated with a decrease in hemoglobin of ≥2 g/dL, a transfusion of ≥2 units of packed red blood cells or whole blood, bleeding at a critical site, or with a fatal outcome. | 395 (3.6) | 386 (3.5) | 1.04 (0.90, 1.20) |
| Intracranial Hemorrhage (ICH) Intracranial bleeding events included intraparenchymal, intraventricular, subdural, subarachnoid and/or epidural hematoma. | 55 (0.5) | 84 (0.7) | 0.67 (0.47, 0.93) |
| Hemorrhagic Stroke Hemorrhagic stroke in this table specifically refers to non-traumatic intraparenchymal and/or intraventricular hematoma in patients on treatment plus 2 days. | 36 (0.3) | 58 (0.5) | 0.63 (0.42, 0.96) |
| Other ICH | 19 (0.2) | 26 (0.2) | 0.74 (0.41, 1.34) |
| Gastrointestinal (GI) Gastrointestinal bleeding events included upper GI, lower GI, and rectal bleeding. | 221 (2.0) | 140 (1.2) | 1.61 (1.30, 1.99) |
| Fatal Bleeding Fatal bleeding is adjudicated death with the primary cause of death from bleeding. | 27 (0.2) | 55 (0.5) | 0.50 (0.31, 0.79) |
| ICH | 24 (0.2) | 42 (0.4) | 0.58 (0.35, 0.96) |
| Non-intracranial | 3 (0.0) | 13 (0.1) | 0.23 (0.07, 0.82) |
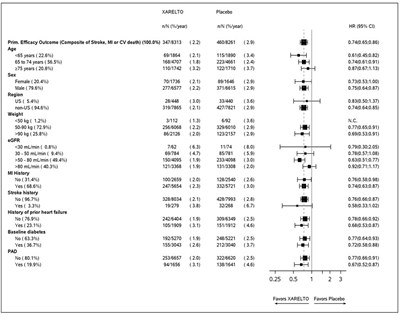
Figure 1 shows the risk of major bleeding events across major subgroups.
Figure 1: Risk of Major Bleeding Events by Baseline Characteristics in ROCKET AF – On Treatment Plus 2 Days
Note: The figure above presents effects in various subgroups all of which are baseline characteristics and all of which were pre-specified (diabetic status was not pre-specified in the subgroup, but was a criterion for the CHADS2 score). The 95% confidence limits that are shown do not take into account how many comparisons were made, nor do they reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted.
Treatment of Deep Vein Thrombosis (DVT) and/or Pulmonary Embolism (PE)
EINSTEIN DVT and EINSTEIN PE Studies
In the pooled analysis of the EINSTEIN DVT and EINSTEIN PE clinical studies, the most frequent adverse reactions leading to permanent drug discontinuation were bleeding events, with XARELTO vs. enoxaparin/Vitamin K antagonist (VKA) incidence rates of 1.7% vs. 1.5%, respectively. The mean duration of treatment was 208 days for XARELTO-treated patients and 204 days for enoxaparin/VKA-treated patients.
Table 2 shows the number of patients experiencing major bleeding events in the pooled analysis of the EINSTEIN DVT and EINSTEIN PE studies.
| Parameter | XARELTO Treatment schedule in EINSTEIN DVT and EINSTEIN PE studies: XARELTO 15 mg twice daily for 3 weeks followed by 20 mg once daily; enoxaparin/VKA [enoxaparin: 1 mg/kg twice daily, VKA: individually titrated doses to achieve a target INR of 2.5 (range: 2.0–3.0)] N=4130 n (%) | Enoxaparin/VKA N=4116 n (%) |
|---|---|---|
| Major bleeding event | 40 (1.0) | 72 (1.7) |
| Fatal bleeding | 3 (<0.1) | 8 (0.2) |
| Intracranial | 2 (<0.1) | 4 (<0.1) |
| Non-fatal critical organ bleeding | 10 (0.2) | 29 (0.7) |
| Intracranial Treatment-emergent major bleeding events with at least >2 subjects in any pooled treatment group | 3 (<0.1) | 10 (0.2) |
| Retroperitoneal | 1 (<0.1) | 8 (0.2) |
| Intraocular | 3 (<0.1) | 2 (<0.1) |
| Intra-articular | 0 | 4 (<0.1) |
| Non-fatal non-critical organ bleeding Major bleeding which is not fatal or in a critical organ, but resulting in a decrease in Hb ≥ 2 g/dL and/or transfusion of ≥2 units of whole blood or packed red blood cells | 27 (0.7) | 37 (0.9) |
| Decrease in Hb ≥ 2 g/dL | 28 (0.7) | 42 (1.0) |
| Transfusion of ≥2 units of whole blood or packed red blood cells | 18 (0.4) | 25 (0.6) |
| Clinically relevant non-major bleeding | 357 (8.6) | 357 (8.7) |
| Any bleeding | 1169 (28.3) | 1153 (28.0) |
Reduction in the Risk of Recurrence of DVT and/or PE
EINSTEIN CHOICE Study
In the EINSTEIN CHOICE clinical study, the most frequent adverse reactions associated with permanent drug discontinuation were bleeding events, with incidence rates of 1% for XARELTO 10 mg, 2% for XARELTO 20 mg, and 1% for acetylsalicylic acid (aspirin) 100 mg. The mean duration of treatment was 293 days for XARELTO 10 mg-treated patients and 286 days for aspirin 100 mg-treated patients.
Table 3 shows the number of patients experiencing bleeding events in the EINSTEIN CHOICE study.
| Parameter | XARELTO Treatment schedule: XARELTO 10 mg once daily or aspirin 100 mg once daily. 10 mg N=1127 n (%) | Acetylsalicylic Acid (aspirin) N=1131 n (%) |
|---|---|---|
| Major bleeding event | 5 (0.4) | 3 (0.3) |
| Fatal bleeding | 0 | 1 (<0.1) |
| Non-fatal critical organ bleeding | 2 (0.2) | 1 (<0.1) |
| Non-fatal non-critical organ bleeding Major bleeding which is not fatal or in a critical organ, but resulting in a decrease in Hb ≥ 2 g/dL and/or transfusion of ≥ 2 units of whole blood or packed red blood cells. | 3 (0.3) | 1 (<0.1) |
| Clinically relevant non-major (CRNM) bleeding Bleeding which was clinically overt, did not meet the criteria for major bleeding, but was associated with medical intervention, unscheduled contact with a physician, temporary cessation of treatment, discomfort for the patient, or impairment of activities of daily life. | 22 (2.0) | 20 (1.8) |
| Any bleeding | 151 (13.4) | 138 (12.2) |
In the EINSTEIN CHOICE study, there was an increased incidence of bleeding, including major and CRNM bleeding in the XARELTO 20 mg group compared to the XARELTO 10 mg or aspirin 100 mg groups.
Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
In the RECORD clinical trials, the overall incidence rate of adverse reactions leading to permanent treatment discontinuation was 3.7% with XARELTO.
The rates of major bleeding events and any bleeding events observed in patients in the RECORD clinical trials are shown in Table 4.
| XARELTO 10 mg | Enoxaparin Includes the placebo-controlled period for RECORD 2, enoxaparin dosing was 40 mg once daily (RECORD 1–3) | |
|---|---|---|
| Total treated patients | N=4487 n (%) | N=4524 n (%) |
| Major bleeding event | 14 (0.3) | 9 (0.2) |
| Fatal bleeding | 1 (<0.1) | 0 |
| Bleeding into a critical organ | 2 (<0.1) | 3 (0.1) |
| Bleeding that required re-operation | 7 (0.2) | 5 (0.1) |
| Extra-surgical site bleeding requiring transfusion of >2 units of whole blood or packed cells | 4 (0.1) | 1 (<0.1) |
| Any bleeding event Includes major bleeding events | 261 (5.8) | 251 (5.6) |
| Hip Surgery Studies | N=3281 n (%) | N=3298 n (%) |
| Major bleeding event | 7 (0.2) | 3 (0.1) |
| Fatal bleeding | 1 (<0.1) | 0 |
| Bleeding into a critical organ | 1 (<0.1) | 1 (<0.1) |
| Bleeding that required re-operation | 2 (0.1) | 1 (<0.1) |
| Extra-surgical site bleeding requiring transfusion of >2 units of whole blood or packed cells | 3 (0.1) | 1 (<0.1) |
| Any bleeding event | 201 (6.1) | 191 (5.8) |
| Knee Surgery Study | N=1206 n (%) | N=1226 n (%) |
| Major bleeding event | 7 (0.6) | 6 (0.5) |
| Fatal bleeding | 0 | 0 |
| Bleeding into a critical organ | 1 (0.1) | 2 (0.2) |
| Bleeding that required re-operation | 5 (0.4) | 4 (0.3) |
| Extra-surgical site bleeding requiring transfusion of >2 units of whole blood or packed cells | 1 (0.1) | 0 |
| Any bleeding event | 60 (5.0) | 60 (4.9) |
Following XARELTO treatment, the majority of major bleeding complications (≥60%) occurred during the first week after surgery.
Other Adverse Reactions
Non-hemorrhagic adverse reactions reported in ≥1% of XARELTO-treated patients in the EINSTEIN DVT and EINSTEIN PE studies are shown in Table 5.
| Body System Adverse Reaction | ||
|---|---|---|
| EINSTEIN DVT Study | XARELTO 20 mg N=1718 n (%) | Enoxaparin/VKA N=1711 n (%) |
| Gastrointestinal disorders | ||
| Abdominal pain | 46 (2.7) | 25 (1.5) |
| General disorders and administration site conditions | ||
| Fatigue | 24 (1.4) | 15 (0.9) |
| Musculoskeletal and connective tissue disorders | ||
| Back pain | 50 (2.9) | 31 (1.8) |
| Muscle spasm | 23 (1.3) | 13 (0.8) |
| Nervous system disorders | ||
| Dizziness | 38 (2.2) | 22 (1.3) |
| Psychiatric disorders | ||
| Anxiety | 24 (1.4) | 11 (0.6) |
| Depression | 20 (1.2) | 10 (0.6) |
| Insomnia | 28 (1.6) | 18 (1.1) |
| EINSTEIN PE Study | XARELTO 20 mg N=2412 n (%) | Enoxaparin/VKA N=2405 n (%) |
| Skin and subcutaneous tissue disorders | ||
| Pruritus | 53 (2.2) | 27 (1.1) |
Non-hemorrhagic adverse reactions reported in ≥1% of XARELTO-treated patients in RECORD 1–3 studies are shown in Table 6.
| Body System Adverse Reaction | XARELTO 10 mg N=4487 n (%) | Enoxaparin Includes the placebo-controlled period of RECORD 2, enoxaparin dosing was 40 mg once daily (RECORD 1–3) N=4524 n (%) |
|---|---|---|
| Injury, poisoning and procedural complications | ||
| Wound secretion | 125 (2.8) | 89 (2.0) |
| Musculoskeletal and connective tissue disorders | ||
| Pain in extremity | 74 (1.7) | 55 (1.2) |
| Muscle spasm | 52 (1.2) | 32 (0.7) |
| Nervous system disorders | ||
| Syncope | 55 (1.2) | 32 (0.7) |
| Skin and subcutaneous tissue disorders | ||
| Pruritus | 96 (2.1) | 79 (1.8) |
| Blister | 63 (1.4) | 40 (0.9) |
Other clinical trial experience: In an investigational study of acute medically ill patients being treated with XARELTO 10 mg tablets, cases of pulmonary hemorrhage and pulmonary hemorrhage with bronchiectasis were observed.
Interaction with Combined P-gp and Strong CYP3A4 Inhibitors
Avoid concomitant administration of XARELTO with known combined P-gp and strong CYP3A4 inhibitors (e.g., ketoconazole and ritonavir) [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
Although clarithromycin is a combined P-gp and strong CYP3A4 inhibitor, pharmacokinetic data suggests that no precautions are necessary with concomitant administration with XARELTO as the change in exposure is unlikely to affect the bleeding risk [see Clinical Pharmacology (12.3)].
Interaction with Combined P-gp and Moderate CYP3A4 Inhibitors in Patients with Renal Impairment
XARELTO should not be used in patients with CrCl 15 to <80 mL/min who are receiving concomitant combined P-gp and moderate CYP3A4 inhibitors (e.g., erythromycin) unless the potential benefit justifies the potential risk [see Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)].
Risk Summary
The limited available data on XARELTO in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. Use XARELTO with caution in pregnant patients because of the potential for pregnancy related hemorrhage and/or emergent delivery with an anticoagulant that is not readily reversible. The anticoagulant effect of XARELTO cannot be reliably monitored with standard laboratory testing. Consider the benefits and risks of XARELTO for the mother and possible risks to the fetus when prescribing XARELTO to a pregnant woman [see Warnings and Precautions (5.7)].
Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Pregnancy is a risk factor for venous thromboembolism and that risk is increased in women with inherited or acquired thrombophilias. Pregnant women with thromboembolic disease have an increased risk of maternal complications including pre-eclampsia. Maternal thromboembolic disease increases the risk for intrauterine growth restriction, placental abruption and early and late pregnancy loss.
Fetal/Neonatal Adverse Reactions
Based on the pharmacologic activity of Factor Xa inhibitors and the potential to cross the placenta, bleeding may occur at any site in the fetus and/or neonate.
Labor or Delivery
All patients receiving anticoagulants, including pregnant women, are at risk for bleeding and this risk may be increased during labor or delivery [see Warnings and Precautions (5.7)]. The risk of bleeding should be balanced with the risk of thrombotic events when considering the use of XARELTO in this setting.
Data
Human Data
There are no adequate or well-controlled studies of XARELTO in pregnant women, and dosing for pregnant women has not been established. Post-marketing experience is currently insufficient to determine a rivaroxaban-associated risk for major birth defects or miscarriage. In an in vitro placenta perfusion model, unbound rivaroxaban was rapidly transferred across the human placenta.
Animal Data
Rivaroxaban crosses the placenta in animals. Rivaroxaban increased fetal toxicity (increased resorptions, decreased number of live fetuses, and decreased fetal body weight) when pregnant rabbits were given oral doses of ≥10 mg/kg rivaroxaban during the period of organogenesis. This dose corresponds to about 4 times the human exposure of unbound drug, based on AUC comparisons at the highest recommended human dose of 20 mg/day. Fetal body weights decreased when pregnant rats were given oral doses of 120 mg/kg during the period of organogenesis. This dose corresponds to about 14 times the human exposure of unbound drug. In rats, peripartal maternal bleeding and maternal and fetal death occurred at the rivaroxaban dose of 40 mg/kg (about 6 times maximum human exposure of the unbound drug at the human dose of 20 mg/day).
Risk Summary
Rivaroxaban has been detected in human milk. There are insufficient data to determine the effects of rivaroxaban on the breastfed child or on milk production. Rivaroxaban and/or its metabolites were present in the milk of rats. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for XARELTO and any potential adverse effects on the breastfed infant from XARELTO or from the underlying maternal condition (see Data).
Data
Animal data
Following a single oral administration of 3 mg/kg of radioactive [14C]-rivaroxaban to lactating rats between Day 8 to 10 postpartum, the concentration of total radioactivity was determined in milk samples collected up to 32 hours post-dose. The estimated amount of radioactivity excreted with milk within 32 hours after administration was 2.1% of the maternal dose.
Nonvalvular Atrial Fibrillation
In the ROCKET AF trial, patients with CrCl 30 to 50 mL/min were administered XARELTO 15 mg once daily resulting in serum concentrations of rivaroxaban and clinical outcomes similar to those in patients with better renal function administered XARELTO 20 mg once daily. Patients with CrCl 15 to 30 mL/min were not studied, but administration of XARELTO 15 mg once daily is also expected to result in serum concentrations of rivaroxaban similar to those in patients with normal renal function [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
Patients with End-Stage Renal Disease on Dialysis
Clinical efficacy and safety studies with XARELTO did not enroll patients with end-stage renal disease (ESRD) on dialysis. In patients with ESRD maintained on intermittent hemodialysis, administration of XARELTO 15 mg once daily will result in concentrations of rivaroxaban and pharmacodynamic activity similar to those observed in the ROCKET AF study [see Clinical Pharmacology (12.2, 12.3)]. It is not known whether these concentrations will lead to similar stroke reduction and bleeding risk in patients with ESRD on dialysis as was seen in ROCKET AF.
Treatment of DVT and/or PE and Reduction in the Risk of Recurrence of DVT and/or PE
In the EINSTEIN trials, patients with CrCl values <30 mL/min at screening were excluded from the studies. Avoid the use of XARELTO in patients with CrCl <30 mL/min.
Prophylaxis of DVT Following Hip or Knee Replacement Surgery
The combined analysis of the RECORD 1–3 clinical efficacy studies did not show an increase in bleeding risk for patients with CrCl 30 to 50 mL/min and reported a possible increase in total venous thromboemboli in this population. Observe closely and promptly evaluate any signs or symptoms of blood loss in patients with CrCl 30 to 50 mL/min. Avoid the use of XARELTO in patients with CrCl <30 mL/min.
Specific Populations
Renal Impairment
The relationship between systemic exposure and pharmacodynamic activity of rivaroxaban was altered in subjects with renal impairment relative to healthy control subjects [see Use in Specific Populations (8.6)].
| Measure | Parameter | Creatinine Clearance (mL/min) | ||||
|---|---|---|---|---|---|---|
| 50–79 | 30–49 | 15–29 | ESRD (on dialysis) Separate stand-alone study. | ESRD (post-dialysis) | ||
| PT = Prothrombin time; FXa = Coagulation factor Xa; AUC = Area under the plasma concentration-time curve; AUEC = Area under the effect-time curve | ||||||
| Exposure | AUC | 44 | 52 | 64 | 47 | 56 |
| FXa Inhibition | AUEC | 50 | 86 | 100 | 49 | 33 |
| PT Prolongation | AUEC | 33 | 116 | 144 | 112 | 158 |
Hepatic Impairment
Anti-Factor Xa activity was similar in subjects with normal hepatic function and in mild hepatic impairment (Child-Pugh A class). There is no clear understanding of the impact of hepatic impairment beyond this degree on the coagulation cascade and its relationship to efficacy and safety.
Absorption
The absolute bioavailability of rivaroxaban is dose-dependent. For the 10 mg dose, it is estimated to be 80% to 100% and is not affected by food. XARELTO 10 mg tablets can be taken with or without food. For the 20 mg dose in the fasted state, the absolute bioavailability is approximately 66%. Coadministration of XARELTO with food increases the bioavailability of the 20 mg dose (mean AUC and Cmax increasing by 39% and 76% respectively with food). XARELTO 15 mg and 20 mg tablets should be taken with food [see Dosage and Administration (2.2)].
The maximum concentrations (Cmax) of rivaroxaban appear 2 to 4 hours after tablet intake. The pharmacokinetics of rivaroxaban were not affected by drugs altering gastric pH. Coadministration of XARELTO (30 mg single dose) with the H2-receptor antagonist ranitidine (150 mg twice daily), the antacid aluminum hydroxide/magnesium hydroxide (10 mL) or XARELTO (20 mg single dose) with the PPI omeprazole (40 mg once daily) did not show an effect on the bioavailability and exposure of rivaroxaban (see Figure 3).
Absorption of rivaroxaban is dependent on the site of drug release in the GI tract. A 29% and 56% decrease in AUC and Cmax compared to tablet was reported when rivaroxaban granulate is released in the proximal small intestine. Exposure is further reduced when drug is released in the distal small intestine, or ascending colon. Avoid administration of rivaroxaban distal to the stomach which can result in reduced absorption and related drug exposure.
In a study with 44 healthy subjects, both mean AUC and Cmax values for 20 mg rivaroxaban administered orally as a crushed tablet mixed in applesauce were comparable to that after the whole tablet. However, for the crushed tablet suspended in water and administered via an NG tube followed by a liquid meal, only mean AUC was comparable to that after the whole tablet, and Cmax was 18% lower.
Distribution
Plasma protein binding of rivaroxaban in human plasma is approximately 92% to 95%, with albumin being the main binding component. The steady-state volume of distribution in healthy subjects is approximately 50 L.
Metabolism
Approximately 51% of an orally administered [14C]-rivaroxaban dose was recovered as inactive metabolites in urine (30%) and feces (21%). Oxidative degradation catalyzed by CYP3A4/5 and CYP2J2 and hydrolysis are the major sites of biotransformation. Unchanged rivaroxaban was the predominant moiety in plasma with no major or active circulating metabolites.
Excretion
In a Phase 1 study, following the administration of [14C]-rivaroxaban, approximately one-third (36%) was recovered as unchanged drug in the urine and 7% was recovered as unchanged drug in feces. Unchanged drug is excreted into urine, mainly via active tubular secretion and to a lesser extent via glomerular filtration (approximate 5:1 ratio). Rivaroxaban is a substrate of the efflux transporter proteins P-gp and ABCG2 (also abbreviated Bcrp). Rivaroxaban's affinity for influx transporter proteins is unknown.
Rivaroxaban is a low-clearance drug, with a systemic clearance of approximately 10 L/hr in healthy volunteers following intravenous administration. The terminal elimination half-life of rivaroxaban is 5 to 9 hours in healthy subjects aged 20 to 45 years.
Specific Populations
The effects of level of renal impairment, age, body weight, and level of hepatic impairment on the pharmacokinetics of rivaroxaban are summarized in Figure 2.
Figure 2: Effect of Specific Populations on the Pharmacokinetics of Rivaroxaban
Gender
Gender did not influence the pharmacokinetics or pharmacodynamics of XARELTO.
Race
Healthy Japanese subjects were found to have 20 to 40% on average higher exposures compared to other ethnicities including Chinese. However, these differences in exposure are reduced when values are corrected for body weight.
Elderly
The terminal elimination half-life is 11 to 13 hours in the elderly subjects aged 60 to 76 years [see Use in Specific Populations (8.5)].
Renal Impairment
The safety and pharmacokinetics of single-dose XARELTO (10 mg) were evaluated in a study in healthy subjects [CrCl ≥80 mL/min (n=8)] and in subjects with varying degrees of renal impairment (see Figure 2). Compared to healthy subjects with normal creatinine clearance, rivaroxaban exposure increased in subjects with renal impairment. Increases in pharmacodynamic effects were also observed [see Use in Specific Populations (8.6)].
Hemodialysis in ESRD subjects: Systemic exposure to rivaroxaban administered as a single 15 mg dose in ESRD subjects dosed 3 hours after the completion of a 4-hour hemodialysis session (post-dialysis) is 56% higher when compared to subjects with normal renal function (see Table 7). The systemic exposure to rivaroxaban administered 2 hours prior to a 4-hour hemodialysis session with a dialysate flow rate of 600 mL/min and a blood flow rate in the range of 320 to 400 mL/min is 47% higher compared to those with normal renal function. The extent of the increase is similar to the increase in patients with CrCl 15 to 50 mL/min taking XARELTO 15 mg. Hemodialysis had no significant impact on rivaroxaban exposure. Protein binding was similar (86% to 89%) in healthy controls and ESRD subjects in this study.
Hepatic Impairment
The safety and pharmacokinetics of single-dose XARELTO (10 mg) were evaluated in a study in healthy subjects (n=16) and subjects with varying degrees of hepatic impairment (see Figure 2). No patients with severe hepatic impairment (Child-Pugh C) were studied. Compared to healthy subjects with normal liver function, significant increases in rivaroxaban exposure were observed in subjects with moderate hepatic impairment (Child-Pugh B) (see Figure 2). Increases in pharmacodynamic effects were also observed [see Use in Specific Populations (8.7)].
Drug Interactions
In vitro studies indicate that rivaroxaban neither inhibits the major cytochrome P450 enzymes CYP1A2, 2C8, 2C9, 2C19, 2D6, 2J2, and 3A4 nor induces CYP1A2, 2B6, 2C19, or 3A4. In vitro data also indicates a low rivaroxaban inhibitory potential for P-gp and ABCG2 transporters.
The effects of coadministered drugs on the pharmacokinetics of rivaroxaban exposure are summarized in Figure 3 [see Drug Interactions (7) for dosing recommendations].
Figure 3: Effect of Coadministered Drugs on the Pharmacokinetics of Rivaroxaban
Anticoagulants
In a drug interaction study, single doses of enoxaparin (40 mg subcutaneous) and XARELTO (10 mg) given concomitantly resulted in an additive effect on anti-factor Xa activity. In another study, single doses of warfarin (15 mg) and XARELTO (5 mg) resulted in an additive effect on factor Xa inhibition and PT. Neither enoxaparin nor warfarin affected the pharmacokinetics of rivaroxaban (see Figure 3).
NSAIDs/Aspirin
In ROCKET AF, concomitant aspirin use (almost exclusively at a dose of 100 mg or less) during the double-blind phase was identified as an independent risk factor for major bleeding. NSAIDs are known to increase bleeding, and bleeding risk may be increased when NSAIDs are used concomitantly with XARELTO. Neither naproxen nor aspirin affected the pharmacokinetics of rivaroxaban (see Figure 3).
Clopidogrel
In two drug interaction studies where clopidogrel (300 mg loading dose followed by 75 mg daily maintenance dose) and XARELTO (15 mg single dose) were coadministered in healthy subjects, an increase in bleeding time to 45 minutes was observed in approximately 45% and 30% of subjects in these studies, respectively. The change in bleeding time was approximately twice the maximum increase seen with either drug alone. There was no change in the pharmacokinetics of either drug.
Drug-Disease Interactions with Drugs that Inhibit Cytochrome P450 3A4 Enzymes and Drug Transport Systems
In a pharmacokinetic trial, XARELTO was administered as a single dose in subjects with mild (CrCl = 50 to 79 mL/min) or moderate renal impairment (CrCl = 30 to 49 mL/min) receiving multiple doses of erythromycin (a combined P-gp and moderate CYP3A4 inhibitor). Compared to XARELTO administered alone in subjects with normal renal function (CrCl >80 mL/min), subjects with mild and moderate renal impairment concomitantly receiving erythromycin reported a 76% and 99% increase in AUCinf and a 56% and 64% increase in Cmax, respectively. Similar trends in pharmacodynamic effects were also observed.
EINSTEIN Deep Vein Thrombosis and EINSTEIN Pulmonary Embolism Studies
XARELTO for the treatment of DVT and/or PE was studied in EINSTEIN DVT [NCT00440193] and EINSTEIN PE [NCT00439777], multi-national, open-label, non-inferiority studies comparing XARELTO (at an initial dose of 15 mg twice daily with food for the first three weeks, followed by XARELTO 20 mg once daily with food) to enoxaparin 1 mg/kg twice daily for at least five days with VKA and then continued with VKA only after the target INR (2.0–3.0) was reached. Patients who required thrombectomy, insertion of a caval filter, or use of a fibrinolytic agent and patients with creatinine clearance <30 mL/min, significant liver disease, or active bleeding were excluded from the studies. The intended treatment duration was 3, 6, or 12 months based on investigator's assessment prior to randomization.
A total of 8281 (3449 in EINSTEIN DVT and 4832 in EINSTEIN PE) patients were randomized and followed on study treatment for a mean of 208 days in the XARELTO group and 204 days in the enoxaparin/VKA group. The mean age was approximately 57 years. The population was 55% male, 70% Caucasian, 9% Asian and about 3% Black. About 73% and 92% of XARELTO-treated patients in the EINSTEIN DVT and EINSTEIN PE studies, respectively, received initial parenteral anticoagulant treatment for a median duration of 2 days. Enoxaparin/VKA-treated patients in the EINSTEIN DVT and EINSTEIN PE studies received initial parenteral anticoagulant treatment for a median duration of 8 days. Aspirin was taken as on treatment concomitant antithrombotic medication by approximately 12% of patients in both treatment groups. Patients randomized to VKA had an unadjusted mean percentage of time in the INR target range of 2.0 to 3.0 of 58% in EINSTEIN DVT study and 60% in EINSTEIN PE study, with the lower values occurring during the first month of the study.
In the EINSTEIN DVT and EINSTEIN PE studies, 49% of patients had an idiopathic DVT/PE at baseline. Other risk factors included previous episode of DVT/PE (19%), recent surgery or trauma (18%), immobilization (16%), use of estrogen-containing drug (8%), known thrombophilic conditions (6%), or active cancer (5%).
In the EINSTEIN DVT and EINSTEIN PE studies, XARELTO was demonstrated to be non-inferior to enoxaparin/VKA for the primary composite endpoint of time to first occurrence of recurrent DVT or non-fatal or fatal PE [EINSTEIN DVT HR (95% CI): 0.68 (0.44, 1.04); EINSTEIN PE HR (95% CI): 1.12 (0.75, 1.68)]. In each study the conclusion of non-inferiority was based on the upper limit of the 95% confidence interval for the hazard ratio being less than 2.0.
Table 9 displays the overall results for the primary composite endpoint and its components for EINSTEIN DVT and EINSTEIN PE studies.
| Event | XARELTO 20 mg Treatment schedule in EINSTEIN DVT and EINSTEIN PE studies: XARELTO 15 mg twice daily for 3 weeks followed by 20 mg once daily; enoxaparin/VKA [enoxaparin: 1 mg/kg twice daily, VKA: individually titrated doses to achieve a target INR of 2.5 (range: 2.0–3.0)] | Enoxaparin/VKA | XARELTO vs. Enoxaparin/VKA Hazard Ratio (95% CI) |
|---|---|---|---|
| EINSTEIN DVT Study | N=1731 n (%) | N=1718 n (%) | |
| Primary Composite Endpoint | 36 (2.1) | 51 (3.0) | 0.68 (0.44, 1.04) |
| Death (PE) | 1 (<0.1) | 0 | |
| Death (PE cannot be excluded) | 3 (0.2) | 6 (0.3) | |
| Symptomatic PE and DVT | 1 (<0.1) | 0 | |
| Symptomatic recurrent PE only | 20 (1.2) | 18 (1.0) | |
| Symptomatic recurrent DVT only | 14 (0.8) | 28 (1.6) | |
| EINSTEIN PE Study | N=2419 n (%) | N=2413 n (%) | |
| Primary Composite Endpoint | 50 (2.1) | 44 (1.8) | 1.12 (0.75, 1.68) |
| Death (PE) | 3 (0.1) | 1 (<0.1) | |
| Death (PE cannot be excluded) | 8 (0.3) | 6 (0.2) | |
| Symptomatic PE and DVT | 0 | 2 (<0.1) | |
| Symptomatic recurrent PE only | 23 (1.0) | 20 (0.8) | |
| Symptomatic recurrent DVT only | 18 (0.7) | 17 (0.7) |
Figures 6 and 7 are plots of the time from randomization to the occurrence of the first primary efficacy endpoint event in the two treatment groups in EINSTEIN DVT and EINSTEIN PE studies, respectively.
Figure 6: Time to First Occurrence of the Composite of Recurrent DVT or Non-fatal or Fatal PE by Treatment Group (Intent-to-Treat Population) – EINSTEIN DVT Study
Figure 7: Time to First Occurrence of the Composite of Recurrent DVT or Non-fatal or Fatal PE by Treatment Group (Intent-to-Treat Population) – EINSTEIN PE Study
EINSTEIN CHOICE Study
XARELTO for reduction in the risk of recurrence of DVT and of PE was evaluated in the EINSTEIN CHOICE study [NCT02064439], a multi-national, double-blind, superiority study comparing XARELTO (10 or 20 mg once daily with food) to 100 mg acetylsalicylic acid (aspirin) once daily in patients who had completed 6 to 12 months of anticoagulant treatment for DVT and/or PE following the acute event. The intended treatment duration in the study was up to 12 months. Patients with an indication for continued therapeutic-dose anticoagulation were excluded.
Because the benefit-risk assessment favored the 10 mg dose versus aspirin compared to the 20 mg dose versus aspirin, only the data concerning the 10 mg dose is discussed below.
A total of 2275 patients were randomized and followed on study treatment for a mean of 290 days for the XARELTO and aspirin treatment groups. The mean age was approximately 59 years. The population was 56% male, 70% Caucasian, 14% Asian and 3% Black. In the EINSTEIN CHOICE study, 51% of patients had DVT only, 33% had PE only, and 16% had PE and DVT combined. Other risk factors included idiopathic VTE (43%), previous episode of DVT/PE (17%), recent surgery or trauma (12%), prolonged immobilization (10%), use of estrogen containing drugs (5%), known thrombophilic conditions (6%), Factor V Leiden gene mutation (4%), or active cancer (3%).
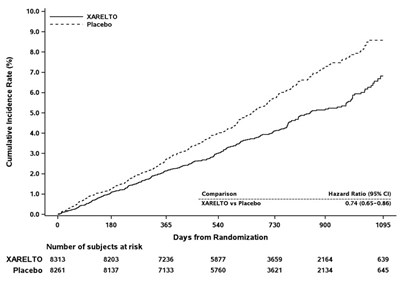
In the EINSTEIN CHOICE study, XARELTO 10 mg was demonstrated to be superior to aspirin 100 mg for the primary composite endpoint of time to first occurrence of recurrent DVT or non-fatal or fatal PE.
Table 10 displays the overall results for the primary composite endpoint and its components.
| Event | XARELTO 10 mg N=1,127 n (%) | Acetylsalicylic Acid (Aspirin) 100 mg N=1,131 n (%) | XARELTO 10 mg vs. Aspirin 100 mg Hazard Ratio (95% CI) |
|---|---|---|---|
| Primary Composite Endpoint | 13 (1.2) | 50 (4.4) | 0.26 (0.14, 0.47) p<0.0001 |
| Symptomatic recurrent DVT | 8 (0.7) | 29 (2.6) | |
| Symptomatic recurrent PE | 5 (0.4) | 19 (1.7) | |
| Death (PE) | 0 | 1 (<0.1) | |
| Death (PE cannot be excluded) | 0 | 1 (<0.1) |
Figure 8 is a plot of the time from randomization to the occurrence of the first primary efficacy endpoint event in the two treatment groups.
Figure 8: Time to First Occurrence of the Composite of Recurrent DVT or Non-fatal or Fatal PE by Treatment Group (Full Analysis Set) – EINSTEIN CHOICE Study
Instructions for Patient Use
Bleeding Risks
Invasive or Surgical Procedures
Instruct patients to inform their healthcare professional that they are taking XARELTO before any invasive procedure (including dental procedures) is scheduled.
Concomitant Medication and Herbals
Advise patients to inform their physicians and dentists if they are taking, or plan to take, any prescription or over-the-counter drugs or herbals, so their healthcare professionals can evaluate potential interactions [see Drug Interactions (7)].
Pregnancy and Pregnancy-Related Hemorrhage
Lactation
Advise patients to discuss with their physician the benefits and risks of XARELTO for the mother and for the child if they are nursing or intend to nurse during anticoagulant treatment [see Use in Specific Populations (8.2)].
Females and Males of Reproductive Potential
Advise patients who can become pregnant to discuss pregnancy planning with their physician [see Use in Specific Populations (8.3)].
Active Ingredient Made in Germany
Finished Product Manufactured by:
Janssen Ortho, LLC
Gurabo, PR 00778
or
Bayer AG
51368 Leverkusen, Germany
Manufactured for:
Janssen Pharmaceuticals, Inc.
Titusville, NJ 08560
Licensed from:
Bayer HealthCare AG
51368 Leverkusen, Germany
© 2011 Janssen Pharmaceutical Companies
1.1 Reduction Of Risk Of Stroke And Systemic Embolism In Nonvalvular Atrial Fibrillation
XARELTO is indicated to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.
There are limited data on the relative effectiveness of XARELTO and warfarin in reducing the risk of stroke and systemic embolism when warfarin therapy is well-controlled [see Clinical Studies (14.1)].
1.2 Treatment Of Deep Vein Thrombosis
XARELTO is indicated for the treatment of deep vein thrombosis (DVT).
1.3 Treatment Of Pulmonary Embolism
XARELTO is indicated for the treatment of pulmonary embolism (PE).
1.4 Reduction In The Risk Of Recurrence Of Deep Vein Thrombosis And/Or Pulmonary Embolism
XARELTO is indicated for the reduction in the risk of recurrence of DVT and/or PE in patients at continued risk for recurrent DVT and/or PE after completion of initial treatment lasting at least 6 months.
1.5 Prophylaxis Of Deep Vein Thrombosis Following Hip Or Knee Replacement Surgery
XARELTO is indicated for the prophylaxis of DVT, which may lead to PE in patients undergoing knee or hip replacement surgery.
2.1 Recommended Dosage
| Indication | Dosage | |
|---|---|---|
| Reduction in Risk of Stroke in Nonvalvular Atrial Fibrillation (2.4) | CrCl >50 mL/min: | 20 mg once daily with the evening meal |
| CrCl 15 to 50 mL/min: | 15 mg once daily with the evening meal | |
| Treatment of DVT (2.5) Treatment of PE (2.5) | 15 mg twice daily with food, for first 21 days | |
| ▼after 21 days, transition to ▼ | ||
| 20 mg once daily with food, for remaining treatment | ||
| Reduction in the Risk of Recurrence of DVT and/or PE in patients at continued risk for DVT and/or PE (2.6) | 10 mg once daily with or without food, after at least 6 months of standard anticoagulant treatment | |
| Prophylaxis of DVT Following Hip or Knee Replacement Surgery (2.7) | Hip replacement: | 10 mg once daily with or without food for 35 days |
| Knee replacement: | 10 mg once daily with or without food for 12 days | |
2.2 Important Food Effect Information
The 15 mg and 20 mg XARELTO tablets should be taken with food, while the 10 mg tablet can be taken with or without food [see Clinical Pharmacology (12.3)].
In the nonvalvular atrial fibrillation efficacy study XARELTO was taken with the evening meal.
2.4 Nonvalvular Atrial Fibrillation
For patients with creatinine clearance (CrCl) >50 mL/min, the recommended dose of XARELTO is 20 mg taken orally once daily with the evening meal. For patients with CrCl 15 to 50 mL/min, the recommended dose is 15 mg once daily with the evening meal [see Use in Specific Populations (8.6)].
2.5 Treatment Of Deep Vein Thrombosis (Dvt) And/Or Pulmonary Embolism (Pe)
The recommended dose of XARELTO for the initial treatment of acute DVT and/or PE is 15 mg taken orally twice daily with food for the first 21 days. After this initial treatment period, the recommended dose of XARELTO is 20 mg taken orally once daily with food, at approximately the same time each day [see Clinical Studies (14.2)].
2.6 Reduction In The Risk Of Recurrence Of Deep Vein Thrombosis (Dvt) And/Or Pulmonary Embolism (Pe)
The recommended dose of XARELTO for the reduction in the risk of recurrence of DVT and/or PE after at least 6 months of standard anticoagulant treatment in patients at continued risk of DVT and/or PE is 10 mg taken orally once daily with or without food [see Clinical Studies (14.3)].
2.7 Prophylaxis Of Deep Vein Thrombosis Following Hip Or Knee Replacement Surgery
The recommended dose of XARELTO is 10 mg taken orally once daily with or without food. The initial dose should be taken 6 to 10 hours after surgery provided that hemostasis has been established [see Dosage and Administration (2.8)].
- For patients undergoing hip replacement surgery, treatment duration of 35 days is recommended.
- For patients undergoing knee replacement surgery, treatment duration of 12 days is recommended.
2.8 Discontinuation For Surgery And Other Interventions
If anticoagulation must be discontinued to reduce the risk of bleeding with surgical or other procedures, XARELTO should be stopped at least 24 hours before the procedure to reduce the risk of bleeding [see Warnings and Precautions (5.2)]. In deciding whether a procedure should be delayed until 24 hours after the last dose of XARELTO, the increased risk of bleeding should be weighed against the urgency of intervention. XARELTO should be restarted after the surgical or other procedures as soon as adequate hemostasis has been established, noting that the time to onset of therapeutic effect is short [see Warnings and Precautions (5.1)]. If oral medication cannot be taken during or after surgical intervention, consider administering a parenteral anticoagulant.
2.9 Missed Dose
If a dose of XARELTO is not taken at the scheduled time, administer the dose as soon as possible on the same day as follows:
- For patients receiving 15 mg twice daily: The patient should take XARELTO immediately to ensure intake of 30 mg XARELTO per day. In this particular instance, two 15 mg tablets may be taken at once. The patient should continue with the regular 15 mg twice daily intake as recommended on the following day.
- For patients receiving 20 mg, 15 mg or 10 mg once daily: The patient should take the missed XARELTO dose immediately.
2.10 Administration Options
For patients who are unable to swallow whole tablets, 10 mg, 15 mg or 20 mg XARELTO tablets may be crushed and mixed with applesauce immediately prior to use and administered orally. After the administration of a crushed XARELTO 15 mg or 20 mg tablet, the dose should be immediately followed by food [see Dosage and Administration (2.2, 2.4, 2.5) and Clinical Pharmacology (12.3)].
3 Dosage Forms And Strengths
- 10 mg tablets: Round, light red, biconvex and film-coated with a triangle pointing down above a "10" marked on one side and "Xa" on the other side
- 15 mg tablets: Round, red, biconvex, and film-coated with a triangle pointing down above a "15" marked on one side and "Xa" on the other side
- 20 mg tablets: Triangle-shaped, dark red, and film-coated with a triangle pointing down above a "20" marked on one side and "Xa" on the other side
4 Contraindications
XARELTO is contraindicated in patients with:
- active pathological bleeding [see Warnings and Precautions (5.2)]
- severe hypersensitivity reaction to XARELTO (e.g., anaphylactic reactions) [see Adverse Reactions (6.2)]
5.1 Increased Risk Of Thrombotic Events After Premature Discontinuation
Premature discontinuation of any oral anticoagulant, including XARELTO, in the absence of adequate alternative anticoagulation increases the risk of thrombotic events. An increased rate of stroke was observed during the transition from XARELTO to warfarin in clinical trials in atrial fibrillation patients. If XARELTO is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant [see Dosage and Administration (2.3, 2.8) and Clinical Studies (14.1)].
5.2 Risk Of Bleeding
XARELTO increases the risk of bleeding and can cause serious or fatal bleeding. In deciding whether to prescribe XARELTO to patients at increased risk of bleeding, the risk of thrombotic events should be weighed against the risk of bleeding.
Promptly evaluate any signs or symptoms of blood loss and consider the need for blood replacement. Discontinue XARELTO in patients with active pathological hemorrhage. The terminal elimination half-life of rivaroxaban is 5 to 9 hours in healthy subjects aged 20 to 45 years.
Concomitant use of other drugs that impair hemostasis increases the risk of bleeding. These include aspirin, P2Y12 platelet inhibitors, other antithrombotic agents, fibrinolytic therapy, non-steroidal anti-inflammatory drugs (NSAIDs) [see Drug Interactions (7.4)], selective serotonin reuptake inhibitors, and serotonin norepinephrine reuptake inhibitors.
Concomitant use of drugs that are known combined P-gp and strong CYP3A4 inhibitors increases rivaroxaban exposure and may increase bleeding risk [see Drug Interactions (7.2)].
5.3 Spinal/Epidural Anesthesia Or Puncture
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal puncture is employed, patients treated with anticoagulant agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis [see Boxed Warning].
To reduce the potential risk of bleeding associated with the concurrent use of XARELTO and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of XARELTO [see Clinical Pharmacology (12.3)]. Placement or removal of an epidural catheter or lumbar puncture is best performed when the anticoagulant effect of XARELTO is low; however, the exact timing to reach a sufficiently low anticoagulant effect in each patient is not known.
An indwelling epidural or intrathecal catheter should not be removed before at least 2 half-lives have elapsed (i.e., 18 hours in young patients aged 20 to 45 years and 26 hours in elderly patients aged 60 to 76 years), after the last administration of XARELTO [see Clinical Pharmacology (12.3)]. The next XARELTO dose should not be administered earlier than 6 hours after the removal of the catheter. If traumatic puncture occurs, delay the administration of XARELTO for 24 hours.
Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/analgesia or lumbar puncture, monitor frequently to detect any signs or symptoms of neurological impairment, such as midline back pain, sensory and motor deficits (numbness, tingling, or weakness in lower limbs), bowel and/or bladder dysfunction. Instruct patients to immediately report if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
5.5 Use In Patients With Hepatic Impairment
No clinical data are available for patients with severe hepatic impairment.
Avoid use of XARELTO in patients with moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment or with any hepatic disease associated with coagulopathy since drug exposure and bleeding risk may be increased [see Use in Specific Populations (8.7)].
5.6 Use With P-Gp And Strong Cyp3a4 Inhibitors Or Inducers
Avoid concomitant use of XARELTO with known combined P-gp and strong CYP3A4 inhibitors [see Drug Interactions (7.2)].
Avoid concomitant use of XARELTO with drugs that are known combined P-gp and strong CYP3A4 inducers [see Drug Interactions (7.3)].
5.7 Risk Of Pregnancy-Related Hemorrhage
In pregnant women, XARELTO should be used only if the potential benefit justifies the potential risk to the mother and fetus. XARELTO dosing in pregnancy has not been studied. The anticoagulant effect of XARELTO cannot be monitored with standard laboratory testing nor readily reversed. Promptly evaluate any signs or symptoms suggesting blood loss (e.g., a drop in hemoglobin and/or hematocrit, hypotension, or fetal distress).
5.8 Patients With Prosthetic Heart Valves
The safety and efficacy of XARELTO have not been studied in patients with prosthetic heart valves. Therefore, use of XARELTO is not recommended in these patients.
5.9 Acute Pe In Hemodynamically Unstable Patients Or Patients Who Require Thrombolysis Or Pulmonary Embolectomy
Initiation of XARELTO is not recommended acutely as an alternative to unfractionated heparin in patients with pulmonary embolism who present with hemodynamic instability or who may receive thrombolysis or pulmonary embolectomy.
6 Adverse Reactions
The following adverse reactions are also discussed in other sections of the labeling:
- Increased risk of stroke after discontinuation in nonvalvular atrial fibrillation [see Boxed Warning and Warnings and Precautions (5.1)]
- Bleeding risk [see Warnings and Precautions (5.2, 5.4, 5.5, 5.6, 5.7)]
- Spinal/epidural hematoma [see Boxed Warning and Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
During clinical development for the approved indications, 18560 patients were exposed to XARELTO. These included 7111 patients who received XARELTO 15 mg or 20 mg orally once daily for a mean of 19 months (5558 for 12 months and 2512 for 24 months) to reduce the risk of stroke and systemic embolism in nonvalvular atrial fibrillation (ROCKET AF); 6962 patients who received XARELTO 15 mg orally twice daily for three weeks followed by 20 mg orally once daily to treat DVT or PE (EINSTEIN DVT, EINSTEIN PE), 10 mg or 20 mg orally once daily (EINSTEIN Extension, EINSTEIN CHOICE) to reduce the risk of recurrence of DVT and/or PE; and 4487 patients who received XARELTO 10 mg orally once daily for prophylaxis of DVT following hip or knee replacement surgery (RECORD 1–3).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of XARELTO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: agranulocytosis, thrombocytopenia
Gastrointestinal disorders: retroperitoneal hemorrhage
Hepatobiliary disorders: jaundice, cholestasis, hepatitis (including hepatocellular injury)
Immune system disorders: hypersensitivity, anaphylactic reaction, anaphylactic shock, angioedema
Nervous system disorders: cerebral hemorrhage, subdural hematoma, epidural hematoma, hemiparesis
Skin and subcutaneous tissue disorders: Stevens-Johnson syndrome
7.1 General Inhibition And Induction Properties
Rivaroxaban is a substrate of CYP3A4/5, CYP2J2, and the P-gp and ATP-binding cassette G2 (ABCG2) transporters. Combined P-gp and strong CYP3A4 inhibitors increase exposure to rivaroxaban and may increase the risk of bleeding. Combined P-gp and strong CYP3A4 inducers decrease exposure to rivaroxaban and may increase the risk of thromboembolic events.
7.3 Drugs That Induce Cytochrome P450 3A4 Enzymes And Drug Transport Systems
Avoid concomitant use of XARELTO with drugs that are combined P-gp and strong CYP3A4 inducers (e.g., carbamazepine, phenytoin, rifampin, St. John's wort) [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
7.4 Anticoagulants And Nsaids/Aspirin
Coadministration of enoxaparin, warfarin, aspirin, clopidogrel and chronic NSAID use may increase the risk of bleeding [see Clinical Pharmacology (12.3)].
Avoid concurrent use of XARELTO with other anticoagulants due to increased bleeding risk unless benefit outweighs risk. Promptly evaluate any signs or symptoms of blood loss if patients are treated concomitantly with aspirin, other platelet aggregation inhibitors, or NSAIDs [see Warnings and Precautions (5.2)].
8.3 Females And Males Of Reproductive Potential
Females of reproductive potential requiring anticoagulation should discuss pregnancy planning with their physician.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
Of the total number of patients in the RECORD 1–3 clinical studies evaluating XARELTO, about 54% were 65 years and over, while about 15% were >75 years. In ROCKET AF, approximately 77% were 65 years and over and about 38% were >75 years. In the EINSTEIN DVT, PE and Extension clinical studies approximately 37% were 65 years and over and about 16% were >75 years. In EINSTEIN CHOICE, approximately 39% were 65 years and over and about 12% were >75 years. In clinical trials the efficacy of XARELTO in the elderly (65 years or older) was similar to that seen in patients younger than 65 years. Both thrombotic and bleeding event rates were higher in these older patients, but the risk-benefit profile was favorable in all age groups [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
8.6 Renal Impairment
In pharmacokinetic studies, compared to healthy subjects with normal creatinine clearance, rivaroxaban exposure increased by approximately 44 to 64% in subjects with renal impairment. Increases in pharmacodynamic effects were also observed [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
In a pharmacokinetic study, compared to healthy subjects with normal liver function, AUC increases of 127% were observed in subjects with moderate hepatic impairment (Child-Pugh B).
The safety or PK of XARELTO in patients with severe hepatic impairment (Child-Pugh C) has not been evaluated [see Clinical Pharmacology (12.3)].
Avoid the use of XARELTO in patients with moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment or with any hepatic disease associated with coagulopathy.
10 Overdosage
Overdose of XARELTO may lead to hemorrhage. Discontinue XARELTO and initiate appropriate therapy if bleeding complications associated with overdosage occur. A specific antidote for rivaroxaban is not available. Rivaroxaban systemic exposure is not further increased at single doses >50 mg due to limited absorption. The use of activated charcoal to reduce absorption in case of XARELTO overdose may be considered. Due to the high plasma protein binding, rivaroxaban is not dialyzable [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)]. Partial reversal of laboratory anticoagulation parameters may be achieved with use of plasma products.
11 Description
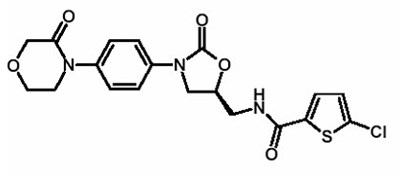
Rivaroxaban, a factor Xa (FXa) inhibitor, is the active ingredient in XARELTO Tablets with the chemical name 5-Chloro-N-({(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl)-2-thiophenecarboxamide. The molecular formula of rivaroxaban is C19H18ClN3O5S and the molecular weight is 435.89. The structural formula is:
Rivaroxaban is a pure (S)-enantiomer. It is an odorless, non-hygroscopic, white to yellowish powder. Rivaroxaban is only slightly soluble in organic solvents (e.g., acetone, polyethylene glycol 400) and is practically insoluble in water and aqueous media.
Each XARELTO tablet contains 10 mg, 15 mg, or 20 mg of rivaroxaban. The inactive ingredients of XARELTO are: croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. Additionally, the proprietary film coating mixture used for XARELTO 10 mg tablets is Opadry® Pink and for XARELTO 15 mg tablets is Opadry® Red, both containing ferric oxide red, hypromellose, polyethylene glycol 3350, and titanium dioxide, and for XARELTO 20 mg tablets is Opadry® II Dark Red, containing ferric oxide red, polyethylene glycol 3350, polyvinyl alcohol (partially hydrolyzed), talc, and titanium dioxide.
12.1 Mechanism Of Action
XARELTO is a selective inhibitor of FXa. It does not require a cofactor (such as Anti-thrombin III) for activity. Rivaroxaban inhibits free FXa and prothrombinase activity. Rivaroxaban has no direct effect on platelet aggregation, but indirectly inhibits platelet aggregation induced by thrombin. By inhibiting FXa, rivaroxaban decreases thrombin generation.
12.2 Pharmacodynamics
Dose-dependent inhibition of FXa activity was observed in humans. Neoplastin® prothrombin time (PT), activated partial thromboplastin time (aPTT) and HepTest® are also prolonged dose-dependently. Anti-factor Xa activity is also influenced by rivaroxaban.
12.6 Qt/Qtc Prolongation
In a thorough QT study in healthy men and women aged 50 years and older, no QTc prolonging effects were observed for XARELTO (15 mg and 45 mg, single-dose).
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
Rivaroxaban was not carcinogenic when administered by oral gavage to mice or rats for up to 2 years. The systemic exposures (AUCs) of unbound rivaroxaban in male and female mice at the highest dose tested (60 mg/kg/day) were 1- and 2-times, respectively, the human exposure of unbound drug at the human dose of 20 mg/day. Systemic exposures of unbound drug in male and female rats at the highest dose tested (60 mg/kg/day) were 2- and 4-times, respectively, the human exposure.
Rivaroxaban was not mutagenic in bacteria (Ames-Test) or clastogenic in V79 Chinese hamster lung cells in vitro or in the mouse micronucleus test in vivo.
No impairment of fertility was observed in male or female rats when given up to 200 mg/kg/day of rivaroxaban orally. This dose resulted in exposure levels, based on the unbound AUC, at least 13 times the exposure in humans given 20 mg rivaroxaban daily.
14.1 Stroke Prevention In Nonvalvular Atrial Fibrillation
The evidence for the efficacy and safety of XARELTO was derived from Rivaroxaban Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonist for the prevention of stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) [NCT00403767], a multi-national, double-blind study comparing XARELTO (at a dose of 20 mg once daily with the evening meal in patients with CrCl >50 mL/min and 15 mg once daily with the evening meal in patients with CrCl 30 to 50 mL/min) to warfarin (titrated to INR 2.0 to 3.0) to reduce the risk of stroke and non-central nervous system (CNS) systemic embolism in patients with nonvalvular atrial fibrillation (AF). Patients had to have one or more of the following additional risk factors for stroke:
- a prior stroke (ischemic or unknown type), transient ischemic attack (TIA) or non-CNS systemic embolism, or
- 2 or more of the following risk factors:
- age ≥75 years,
- hypertension,
- heart failure or left ventricular ejection fraction ≤35%, or
- diabetes mellitus
ROCKET AF was a non-inferiority study designed to demonstrate that XARELTO preserved more than 50% of warfarin's effect on stroke and non-CNS systemic embolism as established by previous placebo-controlled studies of warfarin in atrial fibrillation.
A total of 14264 patients were randomized and followed on study treatment for a median of 590 days. The mean age was 71 years and the mean CHADS2 score was 3.5. The population was 60% male, 83% Caucasian, 13% Asian and 1.3% Black. There was a history of stroke, TIA, or non-CNS systemic embolism in 55% of patients, and 38% of patients had not taken a vitamin K antagonist (VKA) within 6 weeks at time of screening. Concomitant diseases of patients in this study included hypertension 91%, diabetes 40%, congestive heart failure 63%, and prior myocardial infarction 17%. At baseline, 37% of patients were on aspirin (almost exclusively at a dose of 100 mg or less) and few patients were on clopidogrel. Patients were enrolled in Eastern Europe (39%); North America (19%); Asia, Australia, and New Zealand (15%); Western Europe (15%); and Latin America (13%). Patients randomized to warfarin had a mean percentage of time in the INR target range of 2.0 to 3.0 of 55%, lower during the first few months of the study.
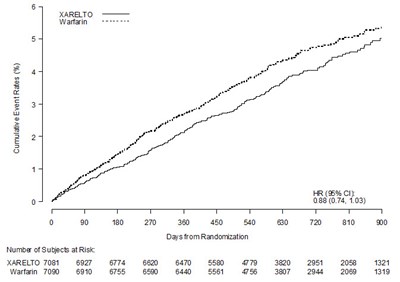
In ROCKET AF, XARELTO was demonstrated non-inferior to warfarin for the primary composite endpoint of time to first occurrence of stroke (any type) or non-CNS systemic embolism [HR (95% CI): 0.88 (0.74, 1.03)], but superiority to warfarin was not demonstrated. There is insufficient experience to determine how XARELTO and warfarin compare when warfarin therapy is well-controlled.
Table 8 displays the overall results for the primary composite endpoint and its components.
| XARELTO | Warfarin | XARELTO vs. Warfarin | |||
|---|---|---|---|---|---|
| Event | N=7081 n (%) | Event Rate (per 100 Pt-yrs) | N=7090 n (%) | Event Rate (per 100 Pt-yrs) | Hazard Ratio (95% CI) |
| Primary Composite Endpoint The primary endpoint was the time to first occurrence of stroke (any type) or non-CNS systemic embolism. Data are shown for all randomized patients followed to site notification that the study would end. | 269 (3.8) | 2.1 | 306 (4.3) | 2.4 | 0.88 (0.74, 1.03) |
| Stroke | 253 (3.6) | 2.0 | 281 (4.0) | 2.2 | |
| Hemorrhagic Stroke Defined as primary hemorrhagic strokes confirmed by adjudication in all randomized patients followed up to site notification | 33 (0.5) | 0.3 | 57 (0.8) | 0.4 | |
| Ischemic Stroke | 206 (2.9) | 1.6 | 208 (2.9) | 1.6 | |
| Unknown Stroke Type | 19 (0.3) | 0.2 | 18 (0.3) | 0.1 | |
| Non-CNS Systemic Embolism | 20 (0.3) | 0.2 | 27 (0.4) | 0.2 | |
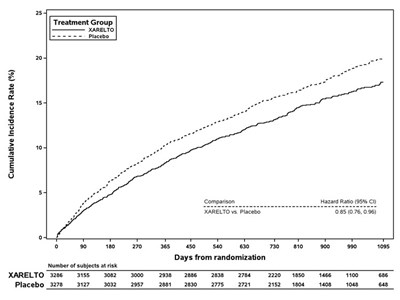
Figure 4 is a plot of the time from randomization to the occurrence of the first primary endpoint event in the two treatment arms.
Figure 4: Time to First Occurrence of Stroke (any type) or Non-CNS Systemic Embolism by Treatment Group (Intent-to-Treat Population)
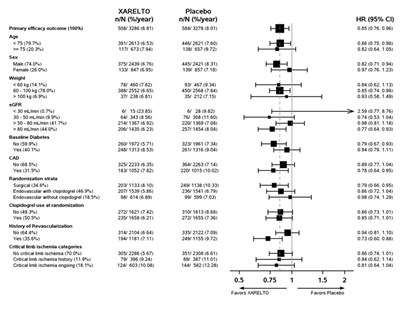
Figure 5 shows the risk of stroke or non-CNS systemic embolism across major subgroups.
| Figure 5: Risk of Stroke or Non-CNS Systemic Embolism by Baseline Characteristics in ROCKET AF Data are shown for all randomized patients followed to site notification that the study would end. |
|---|
The efficacy of XARELTO was generally consistent across major subgroups.
The protocol for ROCKET AF did not stipulate anticoagulation after study drug discontinuation, but warfarin patients who completed the study were generally maintained on warfarin. XARELTO patients were generally switched to warfarin without a period of coadministration of warfarin and XARELTO, so that they were not adequately anticoagulated after stopping XARELTO until attaining a therapeutic INR. During the 28 days following the end of the study, there were 22 strokes in the 4637 patients taking XARELTO vs. 6 in the 4691 patients taking warfarin.
Few patients in ROCKET AF underwent electrical cardioversion for atrial fibrillation. The utility of XARELTO for preventing post-cardioversion stroke and systemic embolism is unknown.
14.4 Prophylaxis Of Deep Vein Thrombosis Following Hip Or Knee Replacement Surgery
XARELTO was studied in 9011 patients (4487 XARELTO-treated, 4524 enoxaparin-treated patients) in the REgulation of Coagulation in ORthopedic Surgery to Prevent DVT and PE, Controlled, Double-blind, Randomized Study of BAY 59-7939 in the Extended Prevention of VTE in Patients Undergoing Elective Total Hip or Knee Replacement (RECORD 1, 2, and 3) [NCT00329628, NCT00332020, NCT00361894] studies.
The two randomized, double-blind, clinical studies (RECORD 1 and 2) in patients undergoing elective total hip replacement surgery compared XARELTO 10 mg once daily starting at least 6 to 8 hours (about 90% of patients dosed 6 to 10 hours) after wound closure versus enoxaparin 40 mg once daily started 12 hours preoperatively. In RECORD 1 and 2, a total of 6727 patients were randomized and 6579 received study drug. The mean age [± standard deviation (SD)] was 63 ± 12.2 (range 18 to 93) years with 49% of patients ≥65 years and 55% of patients were female. More than 82% of patients were White, 7% were Asian, and less than 2% were Black. The studies excluded patients undergoing staged bilateral total hip replacement, patients with severe renal impairment defined as an estimated creatinine clearance <30 mL/min, or patients with significant liver disease (hepatitis or cirrhosis). In RECORD 1, the mean exposure duration (± SD) to active XARELTO and enoxaparin was 33.3 ± 7.0 and 33.6 ± 8.3 days, respectively. In RECORD 2, the mean exposure duration to active XARELTO and enoxaparin was 33.5 ± 6.9 and 12.4 ± 2.9 days, respectively. After Day 13, oral placebo was continued in the enoxaparin group for the remainder of the double-blind study duration. The efficacy data for RECORD 1 and 2 are provided in Table 11.
| RECORD 1 | RECORD 2 | |||||
|---|---|---|---|---|---|---|
| Treatment Dosage and Duration | XARELTO 10 mg once daily | Enoxaparin 40 mg once daily | RRR Relative Risk Reduction; CI = confidence interval ,p-value | XARELTO 10 mg once daily | Enoxaparin Includes the placebo-controlled period of RECORD 2 40 mg once daily | RRR p-value |
| Number of Patients | N=1513 | N=1473 | N=834 | N=835 | ||
| Total VTE | 17 (1.1%) | 57 (3.9%) | 71% (95% CI: 50, 83), p<0.001 | 17 (2.0%) | 70 (8.4%) | 76% (95% CI: 59, 86), p<0.001 |
| Components of Total VTE | ||||||
| Proximal DVT | 1 (0.1%) | 31 (2.1%) | 5 (0.6%) | 40 (4.8%) | ||
| Distal DVT | 12 (0.8%) | 26 (1.8%) | 11 (1.3%) | 43 (5.2%) | ||
| Non-fatal PE | 3 (0.2%) | 1 (0.1%) | 1 (0.1%) | 4 (0.5%) | ||
| Death (any cause) | 4 (0.3%) | 4 (0.3%) | 2 (0.2%) | 4 (0.5%) | ||
| Number of Patients | N=1600 | N=1587 | N=928 | N=929 | ||
| Major VTE Proximal DVT, nonfatal PE or VTE-related death | 3 (0.2%) | 33 (2.1%) | 91% (95% CI: 71, 97), p<0.001 | 6 (0.7%) | 45 (4.8%) | 87% (95% CI: 69, 94), p<0.001 |
| Number of Patients | N=2103 | N=2119 | N=1178 | N=1179 | ||
| Symptomatic VTE | 5 (0.2%) | 11 (0.5%) | 3 (0.3%) | 15 (1.3%) | ||
One randomized, double-blind, clinical study (RECORD 3) in patients undergoing elective total knee replacement surgery compared XARELTO 10 mg once daily started at least 6 to 8 hours (about 90% of patients dosed 6 to 10 hours) after wound closure versus enoxaparin. In RECORD 3, the enoxaparin regimen was 40 mg once daily started 12 hours preoperatively. The mean age (± SD) of patients in the study was 68 ± 9.0 (range 28 to 91) years with 66% of patients ≥65 years. Sixty-eight percent (68%) of patients were female. Eighty-one percent (81%) of patients were White, less than 7% were Asian, and less than 2% were Black. The study excluded patients with severe renal impairment defined as an estimated creatinine clearance <30 mL/min or patients with significant liver disease (hepatitis or cirrhosis). The mean exposure duration (± SD) to active XARELTO and enoxaparin was 11.9 ± 2.3 and 12.5 ± 3.0 days, respectively. The efficacy data are provided in Table 12.
| RECORD 3 | |||
|---|---|---|---|
| Treatment Dosage and Duration | XARELTO 10 mg once daily | Enoxaparin 40 mg once daily | RRR Relative Risk Reduction; CI = confidence interval , p-value |
| Number of Patients | N=813 | N=871 | |
| Total VTE | 79 (9.7%) | 164 (18.8%) | 48% (95% CI: 34, 60), p<0.001 |
| Components of events contributing to Total VTE | |||
| Proximal DVT | 9 (1.1%) | 19 (2.2%) | |
| Distal DVT | 74 (9.1%) | 154 (17.7%) | |
| Non-fatal PE | 0 | 4 (0.5%) | |
| Death (any cause) | 0 | 2 (0.2%) | |
| Number of Patients | N=895 | N=917 | |
| Major VTE Proximal DVT, nonfatal PE or VTE-related death | 9 (1.0%) | 23 (2.5%) | 60% (95% CI: 14, 81), p = 0.024 |
| Number of Patients | N=1206 | N=1226 | |
| Symptomatic VTE | 8 (0.7%) | 24 (2.0%) | |
16 How Supplied/Storage And Handling
XARELTO (rivaroxaban) Tablets are available in the strengths and packages listed below:
- 10 mg tablets are round, light red, biconvex film-coated tablets marked with a triangle pointing down above a "10" on one side, and "Xa" on the other side. The tablets are supplied in the packages listed:
NDC 50458-580-30 Bottle containing 30 tablets NDC 50458-580-90 Bottle containing 90 tablets NDC 50458-580-10 Blister package containing 100 tablets (10 blister cards containing 10 tablets each) - 15 mg tablets are round, red, biconvex film-coated tablets with a triangle pointing down above a "15" marked on one side and "Xa" on the other side. The tablets are supplied in the packages listed:
NDC 50458-578-30 Bottle containing 30 tablets NDC 50458-578-90 Bottle containing 90 tablets NDC 50458-578-10 Blister package containing 100 tablets (10 blister cards containing 10 tablets each) - 20 mg tablets are triangle-shaped, dark red film-coated tablets with a triangle pointing down above a "20" marked on one side and "Xa" on the other side. The tablets are supplied in the packages listed:
NDC 50458-579-30 Bottle containing 30 tablets NDC 50458-579-90 Bottle containing 90 tablets NDC 50458-579-10 Blister package containing 100 tablets (10 blister cards containing 10 tablets each) - Starter Pack for treatment of deep vein thrombosis and treatment of pulmonary embolism:
NDC 50458-584-51 30-day starter blister pack containing 51 tablets: 42 tablets of 15 mg and 9 tablets of 20 mg
Storage And Handling
Store at 25°C (77°F) or room temperature; excursions permitted to 15°–30°C (59°–86°F) [see USP Controlled Room Temperature].
Keep out of the reach of children.
17 Patient Counseling Information
See FDA-approved patient labeling (Medication Guide).
Spl Medguide
| Medication Guide has been approved by the U.S. Food and Drug Administration | Revised: 10/2017 |
| MEDICATION GUIDE XARELTO® (zah-REL-toe) (rivaroxaban) tablets | |
What is the most important information I should know about XARELTO?
Call your doctor or get medical help right away if you develop any of these signs or symptoms of bleeding: | |
| What is XARELTO? XARELTO is a prescription medicine used to:
| |
Do not take XARELTO if you:
| |
Before taking XARELTO, tell your doctor about all of your medical conditions, including if you:
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some of your other medicines may affect the way XARELTO works. Certain medicines may increase your risk of bleeding. See "What is the most important information I should know about XARELTO?" | |
How should I take XARELTO?
| |
What are the possible side effects of XARELTO?
| |
How should I store XARELTO?
| |
| General information about the safe and effective use of XARELTO. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use XARELTO for a condition for which it was not prescribed. Do not give XARELTO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about XARELTO that is written for health professionals. | |
| What are the ingredients in XARELTO? Active ingredient: rivaroxaban Inactive ingredients: croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The proprietary film coating mixture for XARELTO 10 mg tablets is Opadry® Pink and contains: ferric oxide red, hypromellose, polyethylene glycol 3350, and titanium dioxide. The proprietary film coating mixture for XARELTO 15 mg tablets is Opadry® Red and contains: ferric oxide red, hypromellose, polyethylene glycol 3350, and titanium dioxide. The proprietary film coating mixture for XARELTO 20 mg tablets is Opadry® II Dark Red and contains: ferric oxide red, polyethylene glycol 3350, polyvinyl alcohol (partially hydrolyzed), talc, and titanium dioxide. Finished Product Manufactured by: Janssen Ortho, LLC Gurabo, PR 00778 or Bayer AG 51368 Leverkusen, Germany Manufactured for: Janssen Pharmaceuticals, Inc. Titusville, NJ 08560 Licensed from: Bayer HealthCare AG 51368 Leverkusen, Germany © 2011 Janssen Pharmaceutical Companies Trademarks are property of their respective owners. For more information call 1-800-526-7736 or go to www.XARELTO-US.com. | |
Principal Display Panel - 10 Mg Tablet Bottle Label
NDC 50458-580-90
90 Tablets
Xarelto®
(rivaroxaban)
Tablets
10 mg
Dispense the accompanying
Medication Guide to each patient.
Each tablet contains:
rivaroxaban 10 mg
See package insert for full
prescribing information.
Rx only
Principal Display Panel - 15 Mg Tablet Bottle Label
NDC 50458-578-30
30 Tablets
Xarelto®
(rivaroxaban)
Tablets
15 mg
Dispense the accompanying
Medication Guide to each patient.
janssen
Rx only
Principal Display Panel - 20 Mg Tablet Bottle Label
NDC 50458-579-30
30 Tablets
Xarelto®
(rivaroxaban)
Tablets
20 mg
Dispense the accompanying
Medication Guide to each patient.
janssen
Rx only
Principal Display Panel - Kit Carton
Xarelto®
(rivaroxaban) Tablets
NDC 50458-584-51
Rx only
Please see full Prescribing Information, including
BOXED WARNINGS, and Medication Guide inside.
Starter Pack
for treatment of deep vein thrombosis and treatment of pulmonary embolism
Days 1-21
15 mg per tablet, twice daily
42 tablets
Days 22-30
20 mg per tablet, once daily
9 tablets
First 30-day supply
LIFT HERE TO OPEN
* Please review the disclaimer below.