Product Images Invega Hafyera
View Photos of Packaging, Labels & Appearance
- Image - invega 01
- Image - invega 02
- Image - invega 03
- Image - invega 04
- Image - invega 05
- Image - invega 06
- Image - invega 07
- Image - invega 08
- Image - invega 09
- Image - invega 10
- Image - invega 11
- Image - invega 12
- Image - invega 13
- Image - invega 14
- Image - invega 15
- Image - invega 16
- Image - invega 17
- Image - invega 18
- Image - invega 19
- Image - invega 20
- Image - invega 21
- Image - invega 22
- Image - invega 23
- Chemical Structure - invega 24
- Figure 1 - invega 25
- Figure 2 - invega 26
- Figure 3 - invega 27
- Figure 4 - invega 28
- PRINCIPAL DISPLAY PANEL - 3.5 mL Syringe Carton - invega 29
- PRINCIPAL DISPLAY PANEL - 5 mL Syringe Carton - invega 30
Product Label Images
The following 30 images provide visual information about the product associated with Invega Hafyera NDC 50458-611 by Janssen Pharmaceuticals, Inc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Image - invega 03
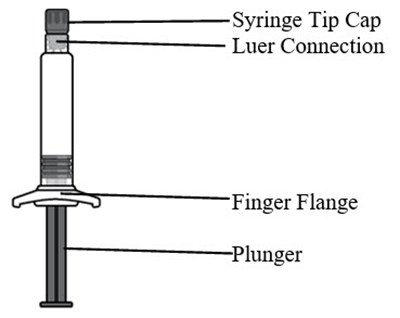
This appears to be a description of components that are commonly found in a syringe. The syringe tip cap is used to cover and protect the tip of the syringe needle. The luer connection is a type of fitting that allows the syringe to be connected to other devices such as needles or IV tubing. The finger flange is a piece that can be grasped between the fingers to help with needle placement and control. The plunger is the component that is pushed to expel liquid from the syringe. These components are essential in the proper use of a syringe.*
Image - invega 05
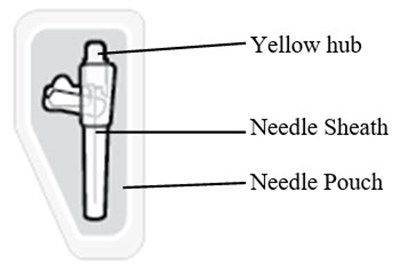
This text suggests the presence of three items: a yellow hub, a needle sheath, and a needle pouch. However, no further description or context is available to evaluate the nature of these items.*
Image - invega 07

The text appears to be a set of instructions related to the use of INVEGA HAFYERA. It suggests that if more than 5 minutes pass before injection, the syringe should be shaken very fast with the tip cap pointing up again for at least 30 seconds to resuspend the medication.*
Image - invega 20

This text provides guidance on injections in the gluteal muscle, reminding the reader to ensure that the entire contents of the syringe have been injected while the needle is still in place.*
Figure 1 - invega 25
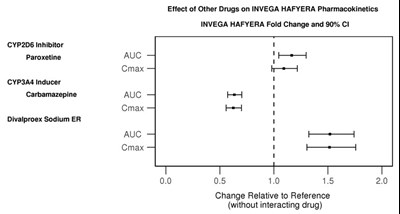
Effect of Other Drugs on INVEGA HAFYERA Pharmacokinetics: The text provides a table showing the fold change and 90% confidence intervals of the pharmacokinetics of INVEGA HAFYERA when taken with other drugs, including a CYP2D6 inhibitor (paroxetine) and a CYP3A4 inducer (carbamazepine and divalproex sodium ER). The table shows the change relative to the reference (without interacting drug) in terms of AUC and Cmax.*
Figure 3 - invega 27
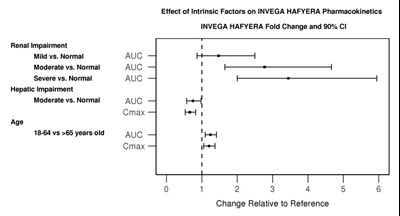
This is a table presenting the effect of intrinsic factors on the pharmacokinetics of INVEGA HAFYERA medication. The factors included in the study are renal and hepatic impairment, and age. The table shows the fold change and 90% CI for each factor compared to the reference. The factors are presented by their severity, i.e., mild, moderate, or severe. The parameters measured are AUC and Cmax. However, there is no numerical data presented in the table.*
Figure 4 - invega 28

This is a graph that compares the relapse rate between two treatments: INVEGA HAFYERA with N=478 and PP3M with N=224. The relapse rate for the former was 50%, while for the latter, it was 40%. The graph shows the time since randomization in days on the X-axis and the percentage of subjects with relapse on the Y-axis.*
PRINCIPAL DISPLAY PANEL - 3.5 mL Syringe Carton - invega 29

This appears to be a description of a prescription medication called INVEGA HAFYERA, which is an extended-release injectable suspension used for gluteal intramuscular injection only. Each injection must be administered only by a healthcare professional, and their dosage is available from the prescribing information. The medication comes in a single-dose prefilled syringe and a needle. The recommended storage temperature is between 20°C to 25°C. There are also warnings and information about how to use the medication, such as shaking it before using.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.





















