Product Images Neupro
View Photos of Packaging, Labels & Appearance
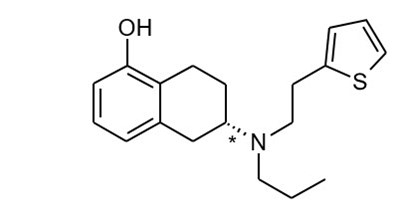
- Chemical Structure - neupro 01
- Figure - neupro 02
- Figure 1 - neupro 03
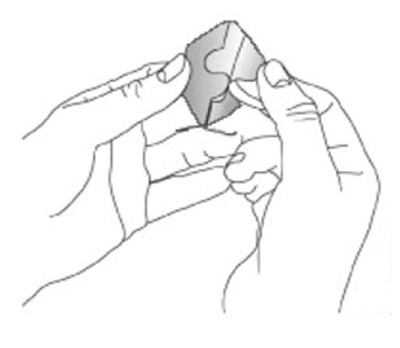
- Figure A - neupro 04
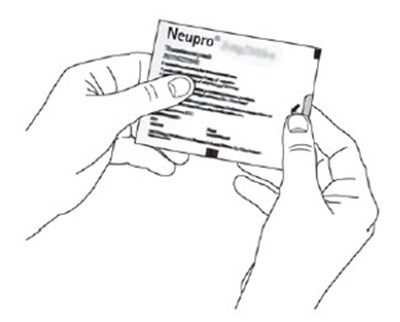
- Figure B - neupro 05
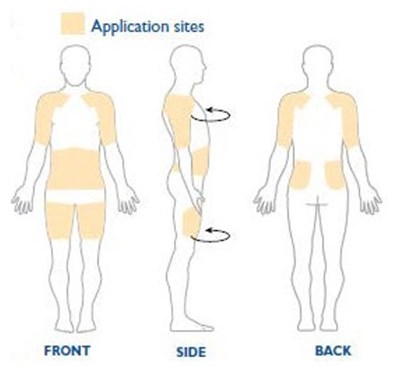
- Figure C - neupro 06
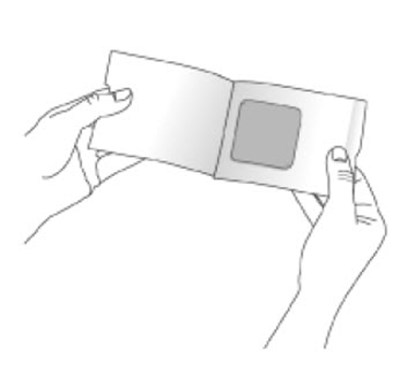
- Figure D - neupro 07
- Figure E - neupro 08
- Figure F - neupro 09
- Figure G - neupro 10
- Figure H - neupro 11
- Figure I - neupro 12
- Figure J - neupro 13
- Figure K - neupro 14
- PRINCIPAL DISPLAY PANEL - 1 mg Pouch Carton - neupro 15
- PRINCIPAL DISPLAY PANEL - 2 mg Pouch Carton - neupro 16
- PRINCIPAL DISPLAY PANEL - 3 mg Pouch Carton - neupro 17
- PRINCIPAL DISPLAY PANEL - 4 mg Pouch Carton - neupro 18
- PRINCIPAL DISPLAY PANEL - 6 mg Pouch Carton - neupro 19
- PRINCIPAL DISPLAY PANEL - 8 mg Pouch Carton - neupro 20
- PRINCIPAL DISPLAY PANEL - Kit Carton - neupro 21
- neupro 22
- neupro 23
- neupro 24
- neupro 25
- neupro 26
- neupro 27
- neupro 28
- neupro 29
- neupro 30
- neupro 31
- neupro 32
Product Label Images
The following 32 images provide visual information about the product associated with Neupro NDC 50474-804 by Ucb, Inc., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1 - neupro 03
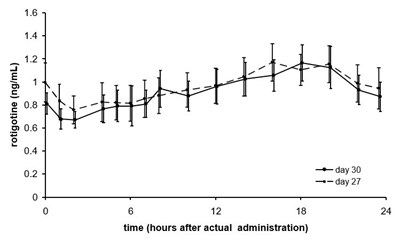
This text likely represents a graph or chart depicting the concentration of rotigotine (in ng/mL) over time (in hours) after administration on day 27 and day 30. The values for rotigotine concentration are given at 6, 12, 18, and 24 hours after administration. No further information is available.*
neupro 26

Neupro is a transdermal system for rotigotine that is used to deliver 1mg/24 hours. It is manufactured by UCB, Inc. and has a registered trademark. The given text includes some packaging information and instructions as well, which might be difficult to interpret due to the erroneous scanning.*
neupro 27

Neupro is a medication provided in the form of rotigotine transdermal system, used to treat Parkinson's disease and restless leg syndrome. It is available in a pack of 30 systems that have 2 mg/24 hours strength each. The medicine is manufactured by UCB, a pharmaceutical company located in Smyrna, Georgia. The text also includes some regulatory information and a trademark reference.*
neupro 28

Neupro® is a transdermal system that contains rotigotine and is available in a strength of 3mg/24 hours. It is a registered trademark of UCB and is indicated for the treatment of Parkinson's disease. The text contains some illegible characters, which can affect the completeness and clarity of the information.*
neupro 29

Neupro® (rotigotine transdermal system) is a registered trademark that comes in the form of a 4 mg/24 hours patch, NDC 50474-804.03, and is manufactured by UCB, Inc. It is a medication used to treat Parkinson's disease and restless legs syndrome. The other text contains some unclear characters and is not useful.*
neupro 30
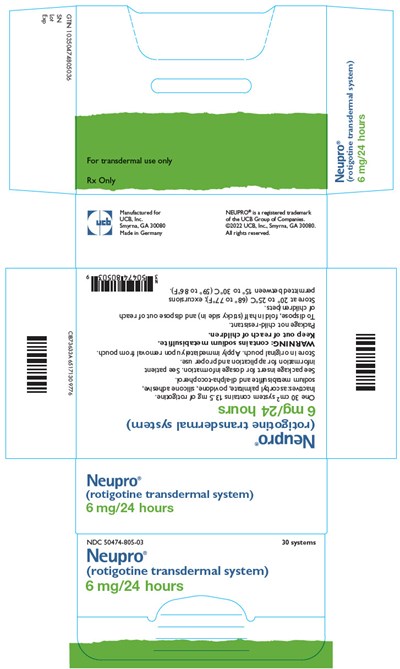
Neupro is a registered trademark of UCB Group of Companies. Neupro (rotigotine transdermal system) is available in 6 mg/24 hours. The package, containing 30 systems, is identified with NDC 50474-805.03. Detailed instructions for use and warnings can be found on the packaging.*
neupro 31

Neupro® (rotigotine transdermal system) is a registered trademark of UCB, Inc. and is made in Germany. This document pertains to an 8mg/24 hours Neupro® product, which comes as a transdermal system. The text also includes some unrecognizable characters.*
neupro 32

Neupro is a transdermal system used for the treatment of Parkinson's disease. It comes in two strengths, 2 mg/24 hours and 4 mg/24 hours. The package contains 17 systems that deliver 2 mg/24 hours each and 87 systems that deliver 4 mg/24 hours each. The transdermal system helps relieve symptoms such as uncontrollable sudden movements and muscle tremors commonly associated with Parkinson's disease. The product is associated with side effects such as vomiting, dizziness, sleepiness, etc. For complete information on dosage and application, consult the package instructions and full prescribing information. The product contains sodium metabisulfite and should be kept out of reach of children.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.