Product Images Bortezomib
View Photos of Packaging, Labels & Appearance
- Chemical Structure - bortezomib 01
- Figure 1 - bortezomib 02
- Figure 2 - bortezomib 03
- Figure 3 - bortezomib 04
- Figure 4 - bortezomib 05
- Figure 5 - bortezomib 06
- Figure 6 - bortezomib 07
- PRINCIPAL DISPLAY PANEL - 3.5 mg Vial Label - bortezomib 08
- PRINCIPAL DISPLAY PANEL - 3.5 mg Vial Carton - bortezomib 09
- PRINCIPAL DISPLAY PANEL INSIDE FLAP (ALL CARTONS) - bortezomib 10
Product Label Images
The following 10 images provide visual information about the product associated with Bortezomib NDC 50742-484 by Ingenus Pharmaceuticals, Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 2 - bortezomib 03
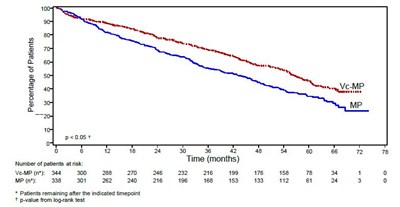
This text appears to be a table displaying the percentage of patients at different time intervals, along with the number of patients at risk. However, the table is not very legible or interpretable without additional context. It is not possible to further interpret the text provided.*
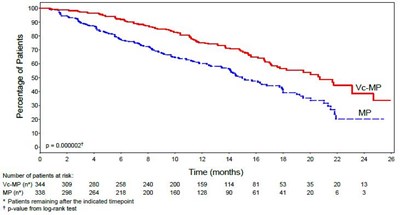
Figure 3 - bortezomib 04

Not available. The text provided is incomplete and does not give enough context to generate a description.*
Figure 4 - bortezomib 05

This appears to be a table displaying the number of patients remaining after a certain timepoint for two treatments - Bortezomib and Dexamethasone. There is also a p-value from a log-rank test. However, there is not enough context to determine what condition or disease is being treated or how the treatments were administered.*
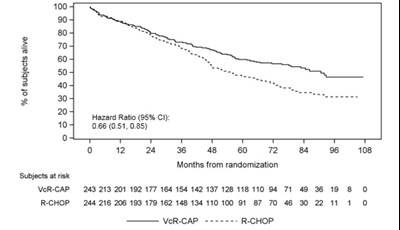
Figure 5 - bortezomib 06

This text seems to indicate the comparison of two chemotherapy regimens (R-CHOP and VcR-CAP) to treat a certain illness in terms of their effectiveness over a period of 48 months. The number of patients receiving each regimen and their progress is also shown. However, without additional context, it is difficult to provide a more detailed description.*
PRINCIPAL DISPLAY PANEL - 3.5 mg Vial Label - bortezomib 08

This is a single-dose vial medicine with NDC code 50742-484-01. It contains Bortezomib and is intended for intravenous or subcutaneous use. The contents of the vial include 3.5 mg bortezomib and 35 mg manitol, with the usual dose provided in the accompanying package insert. The medicine should be stored at 25°C with excursions permitted from 15°C to 30°C, and should be protected from light. The manufacturer is Ingenus Pharmaceuticals, LLC based in Orlando, Florida, and the product was made in China. The text also warns that the medicine is a cytotoxic agent and any unused portion should be discarded.*
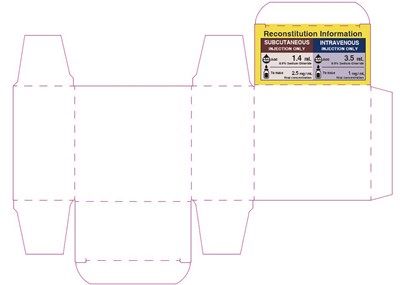
PRINCIPAL DISPLAY PANEL - 3.5 mg Vial Carton - bortezomib 09

This appears to be a label for a medication called "Bortezomib", with dosage information and instructions for use. It is also indicated as a cytotoxic agent, which likely means it is used to treat cancer. The label warns of its potential dangers and advises to only use it as instructed.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.