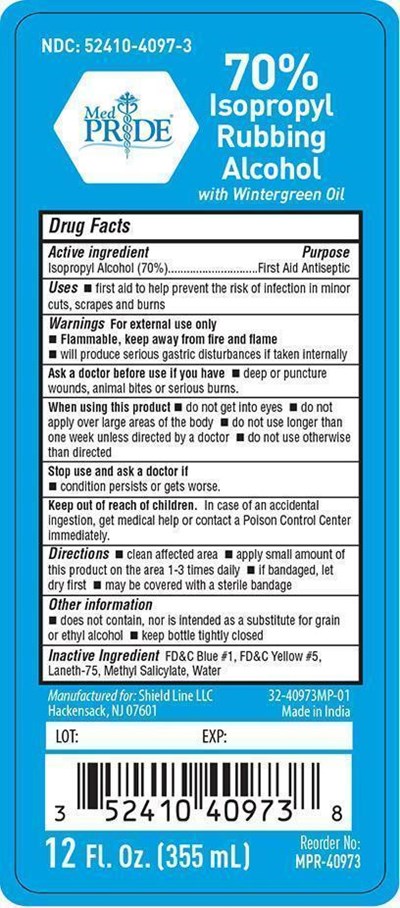
FDA Label for Medpride With Wintergreen Oil
View Indications, Usage & Precautions
Medpride With Wintergreen Oil Product Label
The following document was submitted to the FDA by the labeler of this product Shield Line Llc. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Active Ingredient
Isopropyl Alcohol 70%
Purpose
First Aid Antiseptic
Uses
■ first aid to help prevent risk of infection in minor cuts, scrapes and burns
Warnings
For external use only
■ Flammable, keep away from fire and flame
■ will produce serious gastric disturbances if taken internally
Ask A Doctor Before Use If You Have
■ deep or puncture wounds, animal bites or serious burns
When Using This Product
■ do not get into eyes ■ do not apply over large areas of the body ■ do not use longer than one week unless directed by a doctor
■ do not use otherwise than directed
Stop Use And Ask A Doctor If
■ condition persists or gets worse
Keep Out Of Reach Of Children
In case of an accidental ingestion, get medical help or contact a Poison Control Center immediately
Directions
■ clean affected area ■ apply small amount of this product on the area 1 to 3 times daily
■ if bandaged, let dry first ■ may be covered with a sterile bandage
Other Information
■ does not contain, nor is intended as a substitute for grain or ethyl alcohol ■ keep bottle tightly closed
Inactive Ingredient
FDC Blue 1, FDC Yellow 5, Laneth-75, Methyl Salicylate, Water
Package Label
* Please review the disclaimer below.