Product Images Docetaxel
View Photos of Packaging, Labels & Appearance
Product Label Images
The following 17 images provide visual information about the product associated with Docetaxel NDC 55150-380 by Auromedics Pharma Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-20 mg per 2 mL (10 mg/mL) - Container Label - docetaxel fig11

This is a medication description for a Docetaxel injection manufactured by Indian company AuroMedics Pharma LLC. It is provided in a single-dose vial containing 20mg per 2mL at a concentration of 10mg/mL. It is intended for intravenous use only and is a cytotoxic agent. The NDC code is 55150-378-01. It should be used with caution. The provided text is readable.*
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-20 mg per 2 mL (10 mg/mL) - Container-Carton (1 Vial) - docetaxel fig12

This is a description for a drug called "Docetaxel Injection, USP" distributed by "Seminis" and "Auromedics". It comes in a single-dose vial of 2mL with 20mg of the drug per vial. The drug is intended for intravenous use only and should be checked for concentration prior to preparation. It is important to read the package insert for complete instructions before use. It is a cytotoxic agent, and any unused portions of the vial should be discarded. The drug should be kept out of reach of children.*
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-80 mg per 8 mL (10 mg/mL) - Container Label - docetaxel fig13

This is a prescription drug with NDC code 55150-379-01, containing 10mg Docetaxel USP, 260mg Polysorbate Docetaxel 80 NF, 4mg Anhydrous Citric Acid USP, 23% v/v Dehydrated Alcohol USP, and Polyethylene Glycol 300 NF. It is an injection that should be stored at 20° to 25°C, protected from light. The usual dosage is 80mg per 8mL, but it should only be used intravenously and with caution as it is a cytotoxic agent. It is manufactured by AuroMedics Pharma LLC in India and comes in a sterile 8 mL Multiple-Dose Vial. See package insert for more instructions.*
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-80 mg per 8 mL (10 mg/mL) - Container-Carton (1 Vial) - docetaxel fig14

This is a description of a cytotoxic agent with the brand name "Docetaxel Injection, USP". The drug is available in an 8 mL multi-dose vial, with each mL containing 10mg Docetaxel USP, 260mg Polysorbate 80 N, 4mg Anhydrous Citric Acid USP, 23% uiv Dehydrated Alcohol USP, and 0.5.with Palyethylene Glycol 300 NF. The usual dosage, directions for use, and handling instructions are available on the package insert, and a warning cautions to check the concentration prior to preparation. The product should be stored between 20° to 25°C (68°to TT°F) in its original packaging, and the manufacturer is AuroMedics Pharma LLC.*
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-160 mg per 16 mL (10 mg/mL) - Container Label - docetaxel fig15

This text describes the content and usage instructions of a medication labeled Rxonly NDC 55150-380-01. The medication contains 10mg Docetaxel USP, mixed with other chemicals such as Polysorbate 80 NF and Anhydrous Citric Acid USP. The medication is stored at a temperature range of 20°C to 25°C, and must be protected from light. The package also provides the usual dosage and the manufacturer name, "AuroMedics Pharma LLC".*
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-160 mg per 16 mL (10 mg/mL) - Container-Carton (1 Vial) - docetaxel fig16

This is a description of a medication, Docetaxel Injection, USP, which is a cytotoxic agent. It is used for intravenous administration only and comes in a multiple-dose vial of 16 mL, with a strength of 60 mg per 16 mL (10 mg/mL) or 160 mg per 16 mL (10 mg/mL). The medication is ready to add to an infusion solution, but concentration should be checked before preparation. The package insert should be referred to for complete instructions. The medication should be stored in its original packaging, protected from light, and kept between 20-25°C (68-77°F). The text also states warnings about the medication and the manufacturer, as well as the concentration and composition of the medication.*
Figure 3 - TAX317 Survival K-M Curves - Docetaxel 75 mg/m2 vs. Best Supportive Care - docetaxel fig3

This appears to be the graph of the cumulative probability of overall survival for a certain period. The X-axis represents the survival time in months, ranging from 0 to 18. The Y-axis shows the probability rate from 0 to 1.0, indicating the chance of survival. The Log-rank p value was calculated to be 0.010, which shows a significant difference in survival rates between different groups.*
Figure 4 - TAX320 Survival K-M Curves - Docetaxel 75 mg/m2 vs.Vinorelbine or lfosfamide Control - docetaxel fig4

Figure 5 – Tax327 Survival K-M Curves - docetaxel fig5

This appears to be a chart depicting survival probabilities based on survival time in months (9, 12, 15, 18, 21, 24, 27, and 30). It is not possible to determine further information without additional context.*
Figure 7 – Gastric Cancer Study (TAX325) Survival K-M Curve - docetaxel fig7

This appears to be a graph displaying the survival probability percentage over a period of time in months. However, without more context or additional information, it is difficult to provide a more detailed description.*
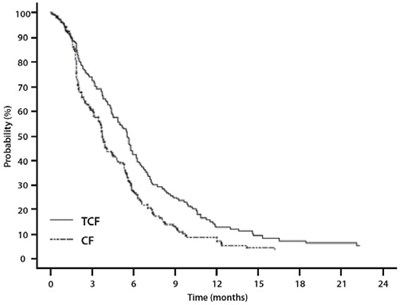
Figure 8 –TAX323 Progression-Free Survival K-M Curve - docetaxel fig8

This is a table showing the probability of progression free survival (PFS) at different time points (12, 18, 24, and 30 months) displayed as percentages for a particular treatment. There is no information about the treatment or the underlying medical condition being treated, other than the PFS time.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.