Product Images Gabapentin
View Photos of Packaging, Labels & Appearance
Product Label Images
The following 10 images provide visual information about the product associated with Gabapentin NDC 55154-8194 by Cardinal Health 107, Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
bag label - D4F21E97 5E55 44C9 9B31 5F79AC2EB512 00

This is a description of Gabapentin Capsules, USP. Each capsule contains 300 mg of Gabapentin USP. The usual dosage is stated to be found in the product insert alongside warnings and precautions. Storage instructions are included, advising to keep the drug at 20° to 25° C (68° to 77° F) and not use if the blister is torn or broken. The package is not child-resistant and is intended for institutional use only. The drug product is contained in this package under NDC # 67877-223 from Ascend Laboratories, LLC. This product is distributed by American Health Packaging, an AmerisourceBergen company. Cardinal health is also listed as a distributor.*
bag label - D4F21E97 5E55 44C9 9B31 5F79AC2EB512 01

This is a description of Gabapentin capsules, USP in a 100mg dosage. It contains 10 capsules per package, and each capsule has 100mg of Gabapentin USP. This medication should be stored at room temperature, and it is not child-resistant. If dispensed for outpatient use, a child-resistant container should be utilized. The package indicates that it can only be used in institutional settings. The distributor of this medication is American Health Packaging, which is an AmerisourceBergen Company, and it is distributed by Cardinal Health. The manufacturer of the drug is Ascend Laboratories, LLC, and the product is labeled with the NDC code 55154-8194-0.*
Cockcroft and Gault Equation - Equation
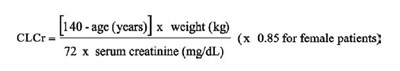
This appears to be a formula for estimating creatinine clearance (CLCr) based on a patient's age and weight. It also factors in the patient's gender using a coefficient of 0.85 for female patients. The formula requires the patient's serum creatinine level measured in mg/dL. However, the text may be incomplete or truncated, as there are missing operators and units.*
Figure 1. Weekly Mean Pain Scores (Observed Cases in ITT Population): Study 1 - Figure1
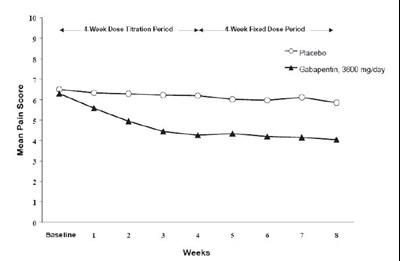
The text describes a chart with the mean pain score for a study conducted in two periods, a titration period of 4 weeks and a fixed dose period of 4 weeks, during which subjects were administered Gabapentin at a daily dosage of 3600 mg/day, and a placebo was used as control. The chart shows a baseline of pain series identified with numbers from 1 to 7, and the pain score ranged from 0-10.*
Figure 2. Weekly Mean Pain Scores (Observed Cases in ITT Population): Study 2 - Figure2
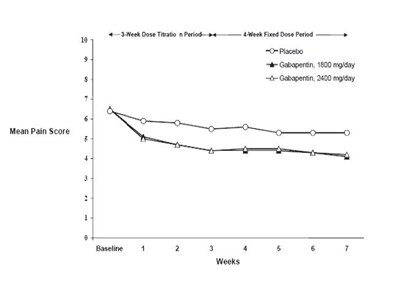
This appears to be a chart or graph showing the results of a study evaluating the effects of Gabapentin at different doses on pain scores over the course of several weeks, with a baseline measurement and measurements taken at the end of each week. The doses used were 1600 mg/day and 2400 mg/day. The chart shows a reduction in pain scores for both Gabapentin doses compared to placebo over the course of the study.*
Figure 3. Proportion of Responders (patients with ≥ 50% reduction in pain score) at Endpoint: Controlled PHN Studies - Figure3
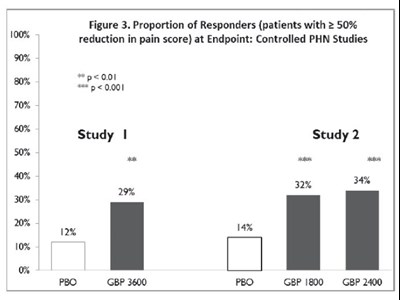
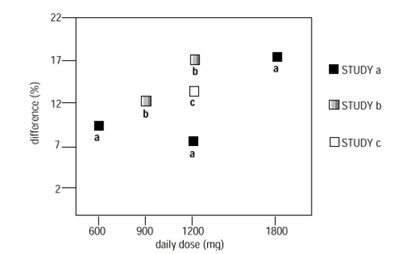
Figure 4. Responder Rate in Patients Receiving gabapentin Expressed as a Difference from Placebo by Dose and Study: Adjunctive Therapy Studies in Patients ≥ 12 Years of Age with Partial Seizures - Figure4
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.