Product Images Prolia
View Photos of Packaging, Labels & Appearance
- Plunger, Safety Guard, Window, Needle Cap, Finger Grip - prolia 01
- Step 1: Remove Grey Needle Cap - prolia 02
- Choose an injection site. The recommended injection sites for Prolia include: the upper arm OR the upper thigh OR the abdomen. - prolia 03
- Insert needle and inject all the liquid subcutaneously. Do not administer into muscle or blood vessel. - prolia 04
- Hold clear finger grip. - prolia 05
- Gently slide green safety guard over needle and lock securely in place. Do not grip green safety guard too firmly when sliding over needle. - prolia 06
- Figure 1. Cumulative Incidence of Hip Fractures Over 3 Years - prolia 07
- prolia 08
- PRINCIPAL DISPLAY PANEL1 x 60 mg Single Use Prefilled SyringeNDC 55513-710-01AMGEN®prolia®(denosumab)60 mg/mL60 mg/mL Injection – For Subcutaneous Use Only.Single Use Prefilled Syringe. Discard unused portion.Sterile Solution – No Preservative.Rx OnlyRefrigerate at 2° to 8°C (36° to 46°F). Do not freeze. Avoid excessive shaking. Protect from direct light and heat.This Product Contains Dry Natural Rubber.Manufactured by: Amgen Inc., Thousand Oaks, CA 91320-1799 - prolia 09
Product Label Images
The following 9 images provide visual information about the product associated with Prolia NDC 55513-710 by Amgen, Inc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Plunger, Safety Guard, Window, Needle Cap, Finger Grip - prolia 01

This text describes different parts of a safety guard such as a green plastic window plunger, a clear plastic finger grip, and a needle cap made of eray rubber. It appears to be related to some sort of medical or safety equipment.*
Choose an injection site. The recommended injection sites for Prolia include: the upper arm OR the upper thigh OR the abdomen. - prolia 03

Insert needle and inject all the liquid subcutaneously. Do not administer into muscle or blood vessel. - prolia 04
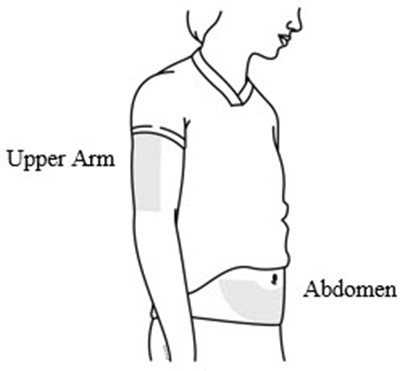
This text provides two body parts: "Upper Arm" and "Abdomen". These are commonly referenced areas of the human body.*
Gently slide green safety guard over needle and lock securely in place. Do not grip green safety guard too firmly when sliding over needle. - prolia 06

PRINCIPAL DISPLAY PANEL1 x 60 mg Single Use Prefilled SyringeNDC 55513-710-01AMGEN®prolia®(denosumab)60 mg/mL60 mg/mL Injection – For Subcutaneous Use Only.Single Use Prefilled Syringe. Discard unused portion.Sterile Solution – No Preservative.Rx OnlyRefrigerate at 2° to 8°C (36° to 46°F). Do not freeze. Avoid excessive shaking. Protect from direct light and heat.This Product Contains Dry Natural Rubber.Manufactured by: Amgen Inc., Thousand Oaks, CA 91320-1799 - prolia 09

* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.



