Product Images Repatha
View Photos of Packaging, Labels & Appearance
- repatha 01
- Figure 1. Estimated Cumulative Incidence of Primary Composite Endpoint Over 3 Years in FOURIER - repatha 02
- Figure 2. Estimated Cumulative Incidence of Key Secondary Composite Endpoint Over 3 Years in FOURIER - repatha 03
- Figure 3. Effect of REPATHA on LDL C in Patients with Hyperlipidemia when Combined with Statins (Mean % Change from Baseline to Week 12 in LAPLACE 2) - repatha 04
- Figure 4. Effect of REPATHA 420 mg Once Monthly on LDL C in Patients with Hyperlipidemia in DESCARTES - repatha 05
- Figure 5. Effect of REPATHA on LDL C in Patients with HeFH (Mean % Change from Baseline to Week 12 in RUTHERFORD 2) - repatha 06
- repatha 07
- Prefilled Cartridge - repatha 08
- On Body Infusor - repatha 09
- Open the on body infusor by swinging the cartridge door to the right. Then, leave the door open. Do not close the cartridge door before the cartridge is loaded. - repatha 10
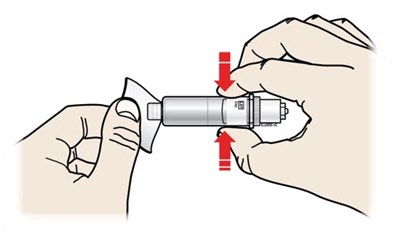
- Inspect the cartridge. - repatha 11
- Clean the cartridge bottom. - repatha 12
- Loadcartridgestraight - repatha 13
- Press downfirmly - repatha 14
- Swing the door to the left. Then, squeeze firmly until it snaps shut. Apply enough pressure when closing the door and make sure there is a “snap” before going to the next step. - repatha 15
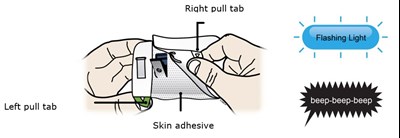
- Peel away both green pull tabs to show the adhesive. The on body infusor is on when the blue status light flashes. - repatha 16
- Stomach area placement - repatha 17
- Thigh placement - repatha 18
- When the blue light flashes, the on body infusor is ready. Keep the stretch (stomach area method only). Hold the loaded on body infusor with the blue light visible, and place it on your skin. You may hear beeps. - repatha 19
- What do I do if the loaded on body infusor status light continuously flashes red and I hear beeps - repatha 20
- Flashing warning light - repatha 21
- beep-beep-beep-beep- beep - repatha 22
- repatha 23
- Do not re-use - repatha 24
- Serial number - repatha 25
- Type BF Applied Part - repatha 26
- Do not use if packaging is damaged - repatha 27
- On-Body Infusor containing420 mg/ 3.5 mL (120 mg/mL) - repatha 28
- Sterilized using ethylene oxide - repatha 29
- Wait at least 30 minutes for the autoinjector to reach room temperature before injecting - repatha 30
- Inspect the Repatha SureClick autoinjector. - repatha 31
- New autoinjector, Alcohol wipes, Cotton ball or gauze pad, Adhesive bandage, Sharps disposal container (see Step 4: Finish) - repatha 32
- Prepare and clean your injection site. - repatha 33
- Pull the orange cap off only when you are ready to inject. - repatha 34
- Do not leave the orange cap off for more than five minutes. This can dry out the medicine. - repatha 35
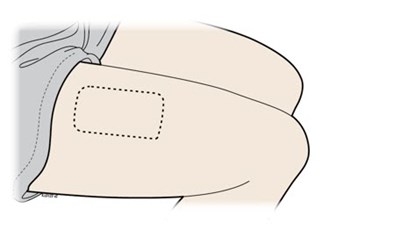
- Thigh:Stretch method - repatha 36
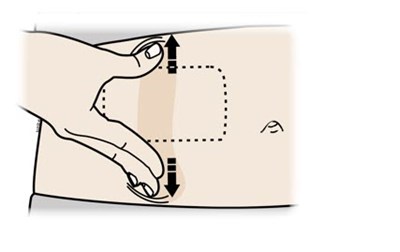
- Stomach or upper arm:Pinch method - repatha 37
- Hold the stretch or pinched skin. With the orange cap off, place autoinjector on the skin at 90 degrees. Do not touch the gray start button yet. - repatha 38
- Firmly push down the autoinjector onto the skin until it stops moving. - repatha 39
- Before use, guide to parts - repatha 40
- Guide to parts- after use - repatha 41
- Remove the Repatha prefilled syringe carton from the refrigerator and wait 30 minutes. - repatha 42
- 1 Rapatha prefilled syringe - repatha 43
- Choose your injection site. - repatha 44
- Clean your injection site with an alcohol wipe. Let your skin dry before injecting - repatha 45
- Remove prefilled syringe from tray. - repatha 46
- Check the medicine and syringe. - repatha 47
- Carefully pull the gray needle cap straight out and away from your body. - repatha 48
- Place the cap in the sharpsdisposal container right away. - repatha 49
- PRINCIPAL DISPLAY PANEL1 x 1 mL Prefilled SyringeNDC 55513-750-01Amgen®RepathaTM (evolocumab)Injection140 mg/mL140 mg/mLPrefilled SyringeFor Subcutaneous Use OnlyStore at 2°C to 8°C (36°F to 46°F). Do NotFreeze or Shake.Store in Carton to Protect from Light.(see side panel for additional storage information)Sterile Solution – No PreservativeSingle UseCAUTION, See package insert for full prescribing information and Instructions for UseThis Product Contains Dry Natural Rubber.Keep out of the sight and reach of childrenRx Only - repatha 50
- PRINCIPAL DISPLAY PANEL1 x 3.5 mL Prefilled Cartridge1 On-Body InfusorNDC 55513-770-01Amgen®Repatha® Pushtronex™ system(evolocumab)On-Body Infusor and Prefilled Cartridge420mg/3.5mL420mg/3.5mLFor Subcutaneous Use OnlySingle-Use OnlySterile Solution – No PreservativeStore refrigerated at 2°C to 8°C (36°F to 46°F). Do Not Freeze or Shake.Store in Carton to Protect from Light.(see side panel for additional storage information)Keep out of the sight and reach of childrenRefer to Instructions for UseDo Not Use if Package is DamagedOn-Body Infusor Sterilized Using Ethylene OxideKeep DrySingle UseType BF Applied PartRelative HumidityRange is 15% to 85%Rx Only - repatha 51
- repatha 52
- repatha 53
- repatha 54
- repatha 55
- repatha 56
- repatha 57
- repatha 58
- repatha 59
- repatha 60
- repatha 61
- repatha 62
- repatha 63
- repatha 64
- repatha 65
- repatha 66
- repatha 67
- repatha 68
- repatha 69
- repatha 70
- repatha 71
- repatha 72
- repatha 73
- repatha 74
- repatha 75
- repatha 76
- repatha 77
- repatha 78
- repatha 79
- repatha 80
- repatha 81
- repatha 82
- repatha 83
- repatha 84
- repatha 85
- repatha 86
Product Label Images
The following 86 images provide visual information about the product associated with Repatha NDC 55513-760 by Amgen, Inc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
repatha 01

This is a table that shows the number of participants at risk over a period of 36 months for a clinical trial that compares the drug Repatha with a placebo for a certain condition. The table shows the number of participants at risk every six months for three years. The first row shows the time in months and the second row shows the number of participants at risk for each treatment group.*
Figure 1. Estimated Cumulative Incidence of Primary Composite Endpoint Over 3 Years in FOURIER - repatha 02

This appears to be a table of data, showing the number of individuals at risk for a placebo group and a group taking a medication called Repatha over a span of 55 months. The table also shows the number of individuals at each time point for both groups.*
Figure 2. Estimated Cumulative Incidence of Key Secondary Composite Endpoint Over 3 Years in FOURIER - repatha 03

Figure 3. Effect of REPATHA on LDL C in Patients with Hyperlipidemia when Combined with Statins (Mean % Change from Baseline to Week 12 in LAPLACE 2) - repatha 04

This text appears to be depicting data in a table format comparing the percent change from a baseline over the course of several weeks for two different treatments, Placebo once monthly and REPATHA 420 mg once monthly. It includes the number of observed participants for each week of the study.*
Figure 4. Effect of REPATHA 420 mg Once Monthly on LDL C in Patients with Hyperlipidemia in DESCARTES - repatha 05

Figure 5. Effect of REPATHA on LDL C in Patients with HeFH (Mean % Change from Baseline to Week 12 in RUTHERFORD 2) - repatha 06

This is a description of the design of a study or clinical trial. It includes information about the number of subjects involved in the study, the baseline week, and the study week. It appears that there were two groups in the study, one group received a placebo and the other group received a drug called EvoMab at a dosage of 420 mg QM.*
repatha 07

This is a description of a medicine cartridge with a white plunger, labeled with the name of the medicine. The text instructs the user not to rotate the plunger. No further information is available.*
Prefilled Cartridge - repatha 08

The text describes the parts and instructions for using a medical device. It includes a cartridge door that should not be closed without a cartridge, a skin status start button, an adhesive light that should not be pressed until ready to inject, a medicine window, and pull tabs. The purpose of the medical device is not specified.*
On Body Infusor - repatha 09

This text is describing a needle cover that is made of adhesive paper. There is also a battery needle inside the strip, which is located under the cover. The cover has both left and right pull tabs.*
Open the on body infusor by swinging the cartridge door to the right. Then, leave the door open. Do not close the cartridge door before the cartridge is loaded. - repatha 10

The text seems to describe a medical device called "On-body infusor" that uses a "prefilled T plastic cover cartridge" to administer medication. It also mentions a "clear tray" that may be used to hold the device or accessories.*
Clean the cartridge bottom. - repatha 12
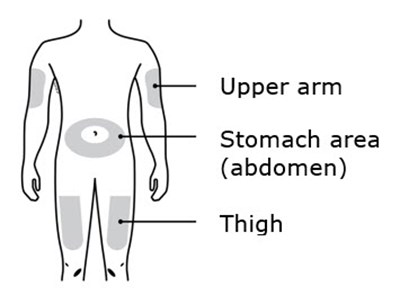
This is a list of body parts - Upper arm, Stomach area (abdomen), and Thigh.*
Swing the door to the left. Then, squeeze firmly until it snaps shut. Apply enough pressure when closing the door and make sure there is a “snap” before going to the next step. - repatha 15
Peel away both green pull tabs to show the adhesive. The on body infusor is on when the blue status light flashes. - repatha 16
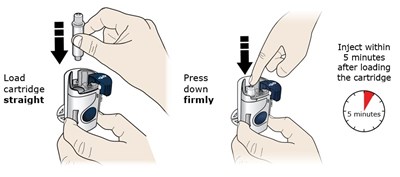
This appears to be instructions for using a cartridge that needs to be loaded and then injected within 5 minutes of loading. The text instructs the user to press down firmly after loading the cartridge. No additional context or product details are available.*
Thigh placement - repatha 18
This document does not provide enough information to generate a useful description. Please provide a different document.*
When the blue light flashes, the on body infusor is ready. Keep the stretch (stomach area method only). Hold the loaded on body infusor with the blue light visible, and place it on your skin. You may hear beeps. - repatha 19
What do I do if the loaded on body infusor status light continuously flashes red and I hear beeps - repatha 20
repatha 23

It is not possible to generate a useful description with the given text as it consists of just a sound description with no context.*
Wait at least 30 minutes for the autoinjector to reach room temperature before injecting - repatha 30

New autoinjector, Alcohol wipes, Cotton ball or gauze pad, Adhesive bandage, Sharps disposal container (see Step 4: Finish) - repatha 32

Do not leave the orange cap off for more than five minutes. This can dry out the medicine. - repatha 35

Hold the stretch or pinched skin. With the orange cap off, place autoinjector on the skin at 90 degrees. Do not touch the gray start button yet. - repatha 38

Remove the Repatha prefilled syringe carton from the refrigerator and wait 30 minutes. - repatha 42

This appears to be a description of a medicine container, with a gray start button, an expiration date, a window, and an orange cap. There is also a yellow safety guard, which seems to indicate that there is a needle inside. The text "0000000 00/14" may be a batch or lot number and expiration date.*
1 Rapatha prefilled syringe - repatha 43

This appears to be a partial label of a medical syringe. It shows a yellow window indicating the completion of an injection with a corresponding expiration date. It also displays a yellow safety guard to cover the needle and an orange cap that should be removed before use.*
Check the medicine and syringe. - repatha 47
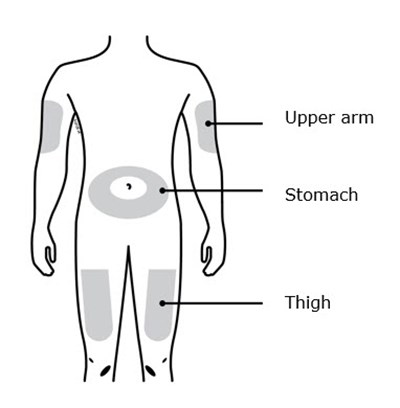
This is a simple list of body parts: the upper arm, stomach, and thigh.*
PRINCIPAL DISPLAY PANEL1 x 1 mL Prefilled SyringeNDC 55513-750-01Amgen®RepathaTM (evolocumab)Injection140 mg/mL140 mg/mLPrefilled SyringeFor Subcutaneous Use OnlyStore at 2°C to 8°C (36°F to 46°F). Do NotFreeze or Shake.Store in Carton to Protect from Light.(see side panel for additional storage information)Sterile Solution – No PreservativeSingle UseCAUTION, See package insert for full prescribing information and Instructions for UseThis Product Contains Dry Natural Rubber.Keep out of the sight and reach of childrenRx Only - repatha 50

PRINCIPAL DISPLAY PANEL1 x 3.5 mL Prefilled Cartridge1 On-Body InfusorNDC 55513-770-01Amgen®Repatha® Pushtronex™ system(evolocumab)On-Body Infusor and Prefilled Cartridge420mg/3.5mL420mg/3.5mLFor Subcutaneous Use OnlySingle-Use OnlySterile Solution – No PreservativeStore refrigerated at 2°C to 8°C (36°F to 46°F). Do Not Freeze or Shake.Store in Carton to Protect from Light.(see side panel for additional storage information)Keep out of the sight and reach of childrenRefer to Instructions for UseDo Not Use if Package is DamagedOn-Body Infusor Sterilized Using Ethylene OxideKeep DrySingle UseType BF Applied PartRelative HumidityRange is 15% to 85%Rx Only - repatha 51

repatha 52

The text is readable but it is not enough to provide a useful description. It only mentions the name of a product and a warning about a needle being inside. More context is needed.*
repatha 64

This text is a list of keywords or phrases related to safety equipment or procedures. Specifically, it mentions a yellow safety guard that may contain a needle inside. Without further context or details, it is difficult to determine the specific application or industry for which this equipment is intended.*
repatha 69

This is a list of items related to a medical syringe, including a plunger rod, a syringe barrel, a gray needle, and a needle cap. The text does not provide much context or detail beyond these basic components.*
repatha 70

This is not-available as the text is incomplete and doesn't provide sufficient information to generate a useful description.*
repatha 84
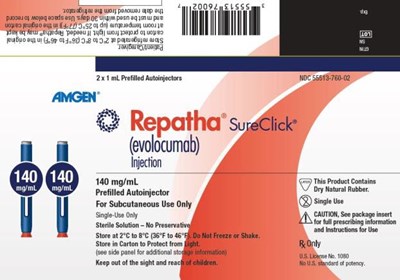
This is a description of a drug called Repatha (evolocumab) which is for subcutaneous use only. It comes in pre-filled autoinjectors of 2x1 mL. The text mentions a sterile solution with no preservatives and indicates caution with package inserts. However, due to the incomplete and fragmented nature of the text obtained by , the overall description is not comprehensive and may not be useful for medical purposes.*
repatha 85

This text appears to be the product information of a medication called Repatha. It comes in a pre-filled syringe with a concentration of 10mg/ml and should be stored between 2-8 degrees Celsius. The medication is intended for subcutaneous use only and instructions are given to protect it from light and to keep it out of reach of children. The text further describes a warning to be cautious when handling the product.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.





















































