Product Images Aimovig
View Photos of Packaging, Labels & Appearance
- Figure 1: Change from Baseline in Monthly Migraine Days in Study 1a - aimovig 01
- Figure 2: Distribution of Change from Baseline in Mean Monthly Migraine Days over Months 4 to 6 by Treatment Group in Study 1 - aimovig 02
- Figure 3: Change from Baseline in Monthly Migraine Days in Study 2a - aimovig 03
- Figure 4: Distribution of Change from Baseline in Monthly Migraine Days at Month 3 by Treatment Group in Study 2 - aimovig 04
- Figure 5: Change from Baseline in Monthly Migraine Days in Study 3a - aimovig 05
- Figure 6: Distribution of Change from Baseline in Monthly Migraine Days at Month 3 by Treatment Group in Study 3 - aimovig 06
- aimovig 07
- Guide to parts - aimovig 08
- Read this before you inject. - aimovig 09
- Pinch method - aimovig 10
- G Hold the stretch or pinch. With the white cap off, place the autoinjector on your skin at 90 degrees. - aimovig 11
- H Firmly push the autoinjector down onto skin until the autoinjector stops moving. - aimovig 12
- I When you are ready to inject, press the purple start button. You will hear a click. - aimovig 13
- J Keep pushing down on your skin. Your injection could take about 15 seconds. When the injection is complete, you may hear or feel a click and the window will turn yellow. - aimovig 14
- K Discard the used autoinjector and the white cap. - aimovig 15
- aimovig 16
- This product contains dry natural rubber - aimovig 17
- Do not reuse - aimovig 18
- CAUTION,consult accompanying documents - aimovig 19
- D Prepare and clean your injection site. - aimovig 20
- E Pull gray needle cap straight out and away from your body, only when you are ready to inject. Do not leave the gray needle cap off for more than 5 minutes. This can dry out the medicine. - aimovig 21
- F Pinch your injection site to create a firm surface. - aimovig 22
- G Hold the pinch. With the gray needle cap off, insert the syringe into your skin at 45 to 90 degrees. - aimovig 23
- H Place your finger on the plunger rod. Using slow and constant pressure, push the plunger rod all the way down until the prefilled syringe stops moving. - aimovig 24
- I When done, release your thumb, and gently lift the syringe off of your skin. - aimovig 25
- J Discard the used syringe and the gray needle cap. - aimovig 26
- This product contains dry natural rubber - aimovig 27
- PRINCIPAL DISPLAY PANEL1 x 70 mg/mL Prefilled AutoinjectorNDC 55513-841-01Rx Onlyaimovig™(erenumab-aooe) Injection70 mg/mL70 mg/mLPrefilled Autoinjector For Subcutaneous Use Only Store at 2°C to 8°C (36°F to 46°F) in original carton to protect from light. Do Not Freeze. Do Not Shake. Discard Any Unused Portion. Keep out of the sight and reach of children. For more information, go to Aimovig.com orcall 1-800-77-AMGEN (1-800-772-6436). CAUTION, ConsultAccompanying Documents Do Not Reuse This Product ContainsDry Natural Rubber.No U.S. standard of potency - aimovig 28
- PRINCIPAL DISPLAY PANEL2 x 70 mg/mL Prefilled AutoinjectorsNDC 55513-841-02Rx Onlyaimovig™(erenumab-aooe) Injection70 mg/mL70 mg/mL140 mg/2 mL70 mg/mLPrefilled Autoinjectors For Subcutaneous Use Only Store at 2°C to 8°C (36°F to 46°F) in original carton to protect from light. Do Not Freeze. Do Not Shake. Discard Any Unused Portion. Keep out of the sight and reach of children. For more information, go to Aimovig.com orcall 1-800-77-AMGEN (1-800-772-6436). CAUTION, ConsultAccompanying Documents Do Not Reuse This Product ContainsDry Natural Rubber.No U.S. standard of potency - aimovig 29
- aimovig 30
- aimovig 31
- aimovig 32
- aimovig 33
- aimovig 34
- aimovig 35
- aimovig 36
- aimovig 37
- aimovig 38
- aimovig 39
- aimovig 40
- aimovig 41
- aimovig 42
- aimovig 43
- aimovig 44
- aimovig 45
- aimovig 46
- aimovig 47
- aimovig 48
- aimovig 49
- aimovig 50
- aimovig 51
- aimovig 52
- aimovig 53
- aimovig 54
- aimovig 55
- aimovig 56
- aimovig 57
- aimovig 58
- aimovig 59
- aimovig 60
- aimovig 61
- aimovig 62
- aimovig 63
- aimovig 64
- aimovig 65
- aimovig 66
Product Label Images
The following 66 images provide visual information about the product associated with Aimovig NDC 55513-840 by Amgen Inc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1: Change from Baseline in Monthly Migraine Days in Study 1a - aimovig 01
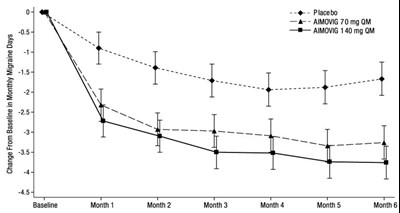
This appears to be a chart or table displaying changes from baseline for a clinical trial involving a placebo and two different doses of a medication called Aimovig (70 mg and 140 mg). The data is organized by month, with columns for baseline and each month up to month 6. There are also two rows labeled "g" and "H" which may be related to the study protocol.*
Figure 2: Distribution of Change from Baseline in Mean Monthly Migraine Days over Months 4 to 6 by Treatment Group in Study 1 - aimovig 02

This is a chart providing the percentage of patients as per the number of days with migraine per month. The chart contains data of two groups, one given a placebo (N=289) and the other given AIMOVIG 70mg QM (N=296). The chart indicates that patients taking AIMOVIG had fewer migraine days per month compared to the placebo group. However, it does not provide any further details or context.*
Figure 3: Change from Baseline in Monthly Migraine Days in Study 2a - aimovig 03

This is a tabular representation of the change in monthly migraine days from the baseline to months 1, 2, and 3. The first row represents the placebo group, and the second row represents the group receiving AIMOVIG 70 mg QM. However, there is not enough information provided to give details beyond this.*
Figure 4: Distribution of Change from Baseline in Monthly Migraine Days at Month 3 by Treatment Group in Study 2 - aimovig 04

Figure 5: Change from Baseline in Monthly Migraine Days in Study 3a - aimovig 05

The text appears to be a chart showing the change in monthly migraine days after using a placebo, AIMOVIG 70 mg QM, and AIMOVIG 140 mg QM over the course of 3 months. It is not possible to decipher the exact meaning of the numbers without more context.*
Figure 6: Distribution of Change from Baseline in Monthly Migraine Days at Month 3 by Treatment Group in Study 3 - aimovig 06

This is a chart that shows the percentage of patients who experienced a reduction in the number of migraine days per month and the percentage of patients who experienced no change or an increase in the number of migraine days per month when taking either placebo, Aimovig 70 mg OM, or Aimovig 140 mg QM. The chart also shows the number of patients who were included in each group.*
aimovig 07

This is a guide to parts for a medical device or medicine administration. It includes instructions on what each part is for before and after use, such as the purple start button, expiration dates, the yellow window, different guards for safety and needles, and when to remove the white cap.*
Pinch method - aimovig 10

This is a list of body parts consisting of the upper arm, stomach area or abdomen, and the thigh.*
G Hold the stretch or pinch. With the white cap off, place the autoinjector on your skin at 90 degrees. - aimovig 11

J Keep pushing down on your skin. Your injection could take about 15 seconds. When the injection is complete, you may hear or feel a click and the window will turn yellow. - aimovig 14

K Discard the used autoinjector and the white cap. - aimovig 15

The Pinch method is a hand gesture often used to assess dehydration in humans. It involves pinching the skin on the back of the hand and observing how quickly it returns to its normal position. If it takes longer than normal, it may be a sign of dehydration.*
E Pull gray needle cap straight out and away from your body, only when you are ready to inject. Do not leave the gray needle cap off for more than 5 minutes. This can dry out the medicine. - aimovig 21

G Hold the pinch. With the gray needle cap off, insert the syringe into your skin at 45 to 90 degrees. - aimovig 23

H Place your finger on the plunger rod. Using slow and constant pressure, push the plunger rod all the way down until the prefilled syringe stops moving. - aimovig 24

This product contains dry natural rubber - aimovig 27

This text appears to be a guide to the different parts of a device or product. It includes descriptions of buttons, windows, expiration dates, safety guards, and caps. The text does not provide enough context to determine what specific product or device this guide applies to.*
PRINCIPAL DISPLAY PANEL1 x 70 mg/mL Prefilled AutoinjectorNDC 55513-841-01Rx Onlyaimovig™(erenumab-aooe) Injection70 mg/mL70 mg/mLPrefilled Autoinjector For Subcutaneous Use Only Store at 2°C to 8°C (36°F to 46°F) in original carton to protect from light. Do Not Freeze. Do Not Shake. Discard Any Unused Portion. Keep out of the sight and reach of children. For more information, go to Aimovig.com orcall 1-800-77-AMGEN (1-800-772-6436). CAUTION, ConsultAccompanying Documents Do Not Reuse This Product ContainsDry Natural Rubber.No U.S. standard of potency - aimovig 28

PRINCIPAL DISPLAY PANEL2 x 70 mg/mL Prefilled AutoinjectorsNDC 55513-841-02Rx Onlyaimovig™(erenumab-aooe) Injection70 mg/mL70 mg/mL140 mg/2 mL70 mg/mLPrefilled Autoinjectors For Subcutaneous Use Only Store at 2°C to 8°C (36°F to 46°F) in original carton to protect from light. Do Not Freeze. Do Not Shake. Discard Any Unused Portion. Keep out of the sight and reach of children. For more information, go to Aimovig.com orcall 1-800-77-AMGEN (1-800-772-6436). CAUTION, ConsultAccompanying Documents Do Not Reuse This Product ContainsDry Natural Rubber.No U.S. standard of potency - aimovig 29

aimovig 30

This appears to be a list of body parts with their respective names: upper arm, stomach area (abdomen), and thigh. It could be useful for identifying specific areas of the body or for medical documentation.*
aimovig 35

The Pinch method is a technique used to estimate body fat percentage by measuring the thickness of skin folds in specific areas of the body, such as the triceps, abdomen, and thighs. By applying pressure to the skin fold, the thickness can be measured and used in a formula to estimate body fat percentage. This method is commonly used in fitness and health assessments to track progress and make adjustments to nutrition and exercise programs.*
aimovig 47

This appears to be a guide to the different parts of a medical syringe, including the plunger rod, finger flange, label, expiration date, and needle. It also mentions the importance of replacing used parts and keeping a needle cap on when not in use.*
aimovig 50

This appears to be a description of a syringe. It includes components such as the barrel, plunger, finger flange, expiration date rod, and a gray medicine needle cap. The syringe also seems to include a label and a possible needle inside the cap.*
aimovig 52

The text describes three body parts: the upper arm, the stomach area (also known as the abdomen), and the thigh.*
aimovig 64

This is a cautionary description about a medical product named "Aimovig" with the active ingredient "erenumab-aooe" in the form of autoinjector for subcutaneous use only. The medical product is to be stored in a refrigerated place between 2°C to 8°C, and the user is advised not to reuse the autoinjector. The product is not meant for children, and the user needs to discard any unused portion. The natural rubber is used in its making, and for more information on this product, the user is directed to visit the website aimovig.com or call 1-800-77-AMGEN (1-800-772-6436).*
aimovig 65

This is a description of a medication called Aimovig. It comes in a prefilled autoinjector with a dose of 140 mg/mL for subcutaneous use only. The medication should be refrigerated at 2°C to 8°C and should not be frozen or shaken. The unused portion should be discarded. The label warns against reusing it and advises consulting accompanying documents. The product contains dry natural rubber. For more information, visit Aimovig.com or call 1-800-77-AMGEN.*
aimovig 66

AIMOVIG is a medication used for subcutaneous injection to help prevent episodic or chronic migraines. The medication comes in a pre-filled syringe that contains 140 mg/mL of erenumab-aooe and should be stored refrigerated between 2°C to 8°C. Before using, the medicine should be allowed to sit at room temperature. The medication is protected from sunlight by keeping the cap on while allowing it to sit. Children should be kept away, and the packaging insert should be referred to for dosage and administration instructions. The text contains additional unrelated content that may be gibberish; however, this information is not available in English.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.








































