Product Images Donepezil Hydrochloride
View Photos of Packaging, Labels & Appearance
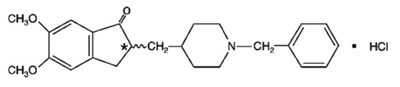
- Structure - donephcltabs figure 01
- Figure 1 - donephcltabs figure 02
- Figure 2 - donephcltabs figure 03
- Figrure 3 - donephcltabs figure 04
- Figure 4 - donephcltabs figure 05
- Figure 5 - donephcltabs figure 06
- Figure 6 - donephcltabs figure 07
- Figure 7 - donephcltabs figure 08
- Figure 8 - donephcltabs figure 09
- Figure 9 - donephcltabs figure 10
- Figure 10 - donephcltabs figure 11
- Container Label - donephcltabs figure 12
- Container Label - donephcltabs figure 13
Product Label Images
The following 13 images provide visual information about the product associated with Donepezil Hydrochloride NDC 55648-311 by Wockhardt Limited, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1 - donephcltabs figure 02
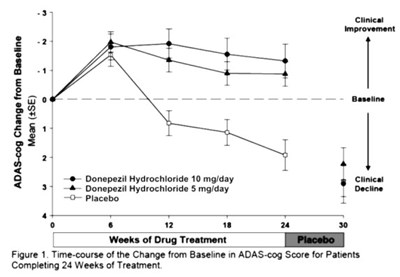
The text describes a graph showing the time-course of change from baseline in ADAS-cog score for patients completing 24 weeks of treatment with Donepezil Hydrochloride, a drug used to improve cognition in Alzheimer's disease patients. The graph compares the drug treatment to a placebo. The text contains data on clinical improvement, decline, baseline and drug dosage.*
Figrure 3 - donephcltabs figure 04
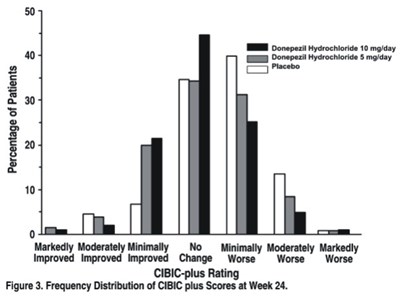
This is an analysis of the frequency distribution of CIBIC-plus ratings for patients taking Donepezil Hydrochloride at different doses over a 24-week period. The CIBIC-plus ratings were categorized based on the level of improvement observed in the patients. The results are presented in a graph labeled Figure 3.*
Figure 4 - donephcltabs figure 05
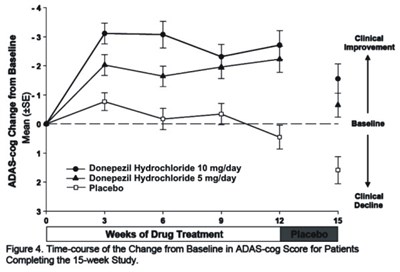
This is a description of a clinical study evaluating the effectiveness of Donepezil Hydrochloride in treating Alzheimer's Disease. Patients were either given 10 mg/day or 5 mg/day of the drug or a placebo. The Time-course of the Change from Baseline in ADAS-cog Score is presented in Figure 4 for patients completing the 15-week study.*
Figure 5 - donephcltabs figure 06

This text provides information on a clinical trial conducted to evaluate the effectiveness of Donepezil Hydrochloride in the treatment of Alzheimer's disease. The trial involved placebo and two treatment groups receiving different doses of Donepezil Hydrochloride per day. The graph shows the change in ADAS-cog scores from baseline for patients in the different groups. The percentage of randomized patients who completed the study and were in each treatment group is also provided.*
Figure 6 - donephcltabs figure 07
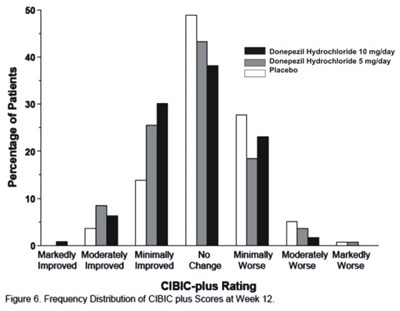
This document appears to be a report of a clinical trial investigating the effects of Donepezil Hydrochloride on patients. The dosage of the drug in the trial was either 10mg/day or 5mg/day. The document includes a chart depicting the distribution of CIBIC plus scores at week 12, which suggests that patients experienced varying degrees of improvement or worsening.*
Figure 7 - donephcltabs figure 08
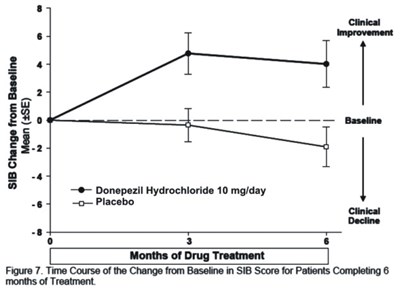
The figure shows the time course of the change from baseline in SIB score for patients completing 6 months of treatment with Donepezil Hydrochloride 10mg/day compared to Placebo. The SIB score represents clinical decline and is depicted as a mean with SE error bars over a 6-month period.*
Figure 10 - donephcltabs figure 11

This is a graph showing the cumulative percentage of patients who complete the 6 months double-blind treatment with Donepezil Hydrochloride at a dosage of 10mg/day. The Vertical axis shows the change from baseline in Activities of Daily Living – Severe Scores (ADCS-ADL). The horizontal axis represents the percentage of patients. The graph indicates that patients who receive Donepezil Hydrochloride showed a significant improvement from the baseline in their ADCS-ADL-Severe Scores.*
Container Label - donephcltabs figure 12

Each tablet of Donepezil HCI contains 5mg of the active ingredient. The dosage and usage instructions are not available in this text and should be found in the accompanying prescribing information. These tablets should be stored in tight containers at a controlled room temperature of 20°-25°C (68°-77°F). The product is available in 30 tablets to be dispensed only by pharmacists with a patient information sheet. This product is manufactured by Wockhardt Limited in Mumbai, India, and distributed by Wockhardt USA LLC in Parsippany, NJ, USA.*
Container Label - donephcltabs figure 13

This is a prescription drug called Donepezil HCI. Each tablet contains 10 mg of Donepezil hydrochloride. It is important to read the accompanying instructions before use. The drug should be stored at 20-25°C in a tight container. The package includes 30 tablets.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.